Nov. 12, 2024
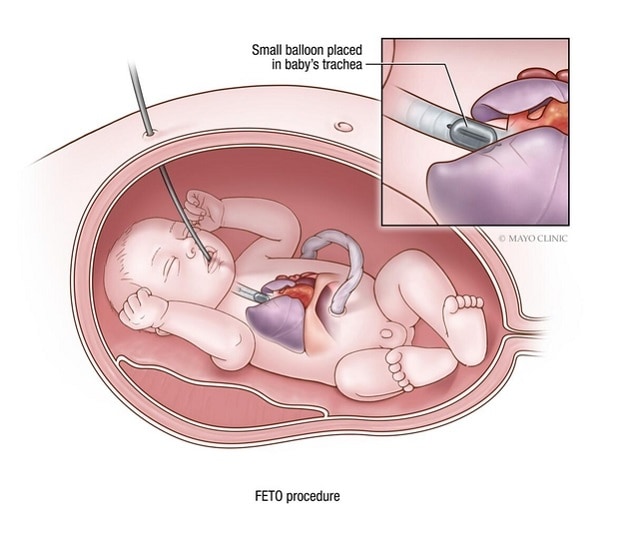 فتق حجابي خلقي
فتق حجابي خلقي
تقنية الإغلاق القصبي بالتنظير الداخلي للجنين لعلاج الفتق الحجابي الخلقي
A North American multisite study of fetoscopic endoluminal tracheal occlusion (FETO) for fetuses with severe, left-sided congenital diaphragmatic hernia (CDH) discovered that a subpopulation of infants who had this procedure received fewer days of extracorporeal membrane oxygenation (ECMO). The study authors say that FETO may, therefore, reduce morbidity in this population.
"Decreased ECMO duration is significant because less ECMO means less exposure to the significant and common risks of ECMO, including risk of thromboembolism and bleeding," says Mauro H. Schenone, M.D., a fetal surgeon at Mayo Clinic in Minnesota and a co-author of the study's findings, which were published in a 2024 issue of Obstetrics & Gynecology.
Eric P. Bergh, M.D., a fetal surgeon at UTHealth Houston who led the study, also believes reduced ECMO duration for infants treated with FETO is an improvement compared with ECMO use needed for infants who are expectantly managed.
North American FETO study findings compared with European study findings
The North American study cohort included patients from 10 North American Fetal Therapy Network centers. Within the cohort, 87 of 89 fetuses (97.8%) had successful FETO. Further, the study found greater — yet not statistically significant — survival rates (69.8% versus 51.8%) for fetuses with this condition who underwent FETO compared with fetuses expectantly managed.
In contrast, the European Tracheal Occlusion to Accelerate Lung Growth (TOTAL) trial found improved survival — a 2.5-fold survival increase — for fetuses with severe, left-sided CDH treated with FETO. This study observed 40% survival for fetuses treated with FETO versus 15% for fetuses expectantly managed.
Therefore, Dr. Schenone and Dr. Bergh believe that further North American research is crucial to better illuminate the survival potential for fetuses with CDH treated with FETO.
"The lack of statistically significant survival benefit may be related to a less strict and uniform diagnostic and inclusion criteria in the U.S. study, which is based on a registry, compared with the mostly European clinical trial," says Dr. Schenone.
Dr. Bergh is hopeful that future FETO studies will find that morbidity improves, especially long-term postprocedural morbidities such as pulmonary hypertension and neurodevelopmental disorders.
Dr. Schenone and Dr. Bergh indicate that when comparing the two FETO studies, it is important to note that postnatal management differs between North America and Europe. The two surgeons say that U.S. postnatal care is more aggressive and uses ECMO more frequently, while care in Europe uses more ventilation and supportive care and less ECMO.
Author perspectives on the North American FETO study
Beyond the frequency of ECMO use, two other North American FETO study findings are important according to Dr. Schenone:
- Feasibility and safety. FETO can be safely performed by a trained fetal surgeon.
- Risks. Compared with expectant management, FETO increases the risk of prematurity.
Dr. Bergh agrees with Dr. Schenone that prematurity is a significant problem accompanying FETO.
According to Dr. Bergh and Dr. Schenone, a limitation of the study is that it was a nonrandomized registry study. However, Dr. Bergh says a strength of their study is participant heterogeneity.
Dr. Bergh also points out that the fetuses in their study had severe CDH-compromised health.
"These fetuses were the sickest of the sick with the highest mortality risk," he says.
Long term, Dr. Bergh says, these extremely ill fetuses with CDH would be at risk of respiratory conditions and eating disorders, exercise intolerance, and need for chronic medical care.
Suggestions for clinical application of findings
Dr. Bergh suggests presenting the option of FETO to patients while being clear about the procedure's unknowns.
"I'd present patients with current data, especially the European TOTAL trial data indicating a survival benefit, in addition to the U.S. data and let them decide whether in utero intervention is right for their families," says Dr. Bergh. "If expectant people and their significant others are willing to move forward with FETO with the knowledge we have today, we offer it."
At the same time, Dr. Bergh says he'd plainly state to patients that currently, FETO has unknowns.
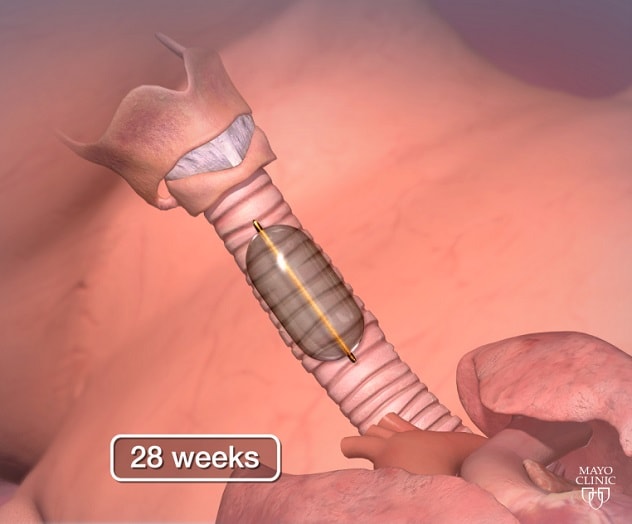 الإغلاق القصبي بالتنظير الداخلي للجنين (FETO) بعد 28 أسبوعًا
الإغلاق القصبي بالتنظير الداخلي للجنين (FETO) بعد 28 أسبوعًا
في جراحة الجنين داخل الرحم كما في الصورة، التي أُجريت هنا في الأسبوع 28 من الحمل، يَستخدم الجراح تقنية التنظير الجنيني الموجّه بالموجات فوق الصوتية. يُدخل الجراح مؤقتًا بالونًا صغيرًا، أقل من 0.5 ملم وهو مفرغ، في مجرى الطفل الهوائي. يُنفَخ البالون إلى حوالي 0.8 ملم، ما يسد القصبة الهوائية للسماح بتراكم السوائل ونمو الرئتين. ثم يُزال البالون بعد عدة أسابيع عندما تنمو الرئتان بما فيه الكفاية للسماح باستمرار نمو الطفل.
"With the data we have today, I'd recommend considering intervention as research for a fetus with CDH and suggest finding a center with experience offering FETO," says Dr. Bergh. "FETO is promising, but we don't fully understand it yet — we are trying to find out if its benefits extend beyond ECMO."
Dr. Schenone also favors an approach of laying out the possibility of FETO for patients whose fetuses have CDH and then letting them make the final decision on the procedure.
He has two suggestions for physicians about fetal CDH monitoring and obtaining specialized care:
- Conduct a full fetal anatomical evaluation. When performing or reviewing an ultrasound at 18 to 20 weeks' gestation, pay close attention to the fetus' chest.
- Refer if CDH is suspected. It is important to make a timely referral if you observe anything questionable in the fetal anatomy.
"Mayo Clinic is ready to help if you have any doubt about chest anatomy following an ultrasound," says Dr. Schenone. "If you delay, you may miss the treatment window, which needs to occur at 27 to 29 weeks. If you miss this window, you have to wait to address the potential CDH until delivery."
Background on fetal severe, left-sided CDH
While CDH is rare, affecting 1 to 4 fetuses per 10,000, it poses a significant mortality risk for the fetus. The condition arises due to a diaphragmatic development issue in which a gap arises through which abdominal organs pass. These organs usurp the space needed for fetal lung development, blocking the fetus' ability to obtain oxygen.
For more information
Bergh E. Fetoscopic endoluminal tracheal occlusion for severe, left-sided congenital diaphragmatic hernia: The North American Fetal Therapy Network Fetoscopic Endoluminal Tracheal Occlusion Consortium experience. Obstetrics & Gynecology. 2024;143:440.
Deprest JA. Randomized trial of fetal surgery for severe left diaphragmatic hernia. The New England Journal of Medicine. 2021;385:107.
Refer a patient to Mayo Clinic.