诊断
目前,帕金森病尚无特定诊断检查。由接受过神经系统状况培训的医生(称为神经内科医生)进行诊断。帕金森病的诊断基于您的病史、症状回顾以及神经系统和体格检查进行。
诊断帕金森病需要花费较长时间。医疗护理专业人员可能会建议您定期前往接受过运动障碍诊疗培训的神经内科医生处复诊,以评估您的状况和症状在一段时间内的变化,并诊断帕金森病。
医疗护理团队可能会要求进行以下的一些检查和程序:
- 体格检查和神经系统检查。 这包括记录您的病史并进行神经系统检查来测试您的思维和心智能力、感觉、协调和反射。
- 血液和实验室检查。 这些检查用于排除可能导致症状的其他状况。
- 成像检查,例如 MRI、脑部超声和 PET 扫描。 这些检查用于排除其他状况。它们对诊断帕金森病没有太大帮助。
- 一种特定单光子发射计算机体层成像(SPECT)扫描,称为多巴胺转运蛋白(DAT)扫描。 虽然该检查可能有助于为怀疑您患帕金森病提供支持,并有助于确定不同类型的震颤,但仍需根据您的症状和神经系统检查结果确诊。大部分患者无需接受 DAT 扫描。
- 基因检测。 如果存在已知的帕金森病家族史或如果您患有早发型疾病,则这项检查可用于检测基因改变。
- 一种短期、低剂量的药物治疗。 您可能会接受用于治疗帕金森病的药物,以观察您是否好转。如果您的症状显著改善,这可能有助于确诊。您必须服用足够剂量才能获得益处,因为低剂量服用一两天无法得到可靠疗效。
- 复诊。 可能需要在一段时间内定期前往接受过运动障碍诊疗培训的神经内科医生处就诊,以确认诊断。
-
α-突触核蛋白检测。 该检测也被称为 α-突触核蛋白种子扩增测定,用于在症状开始前检测帕金森病。α-突触核蛋白团块是帕金森病的标志性体征。医疗护理专业人员可能会在皮肤或脊髓液中检测这种状况。
α-突触核蛋白出现在路易体内。它会形成身体无法分解的团块。团块扩散并损伤脑细胞。
在 2023 年的一项研究中,研究人员检测了 1000 多人的脊髓液,寻找 α-突触核蛋白团块。该检测准确识别出帕金森病患者的概率为 87.7%。该检测也能非常灵敏地检测出存在帕金森病风险的人群。
这次 α-突触核蛋白种子扩增测定是迄今为止规模最大的一项研究。一些研究人员表示,这项研究可能给帕金森病的诊断、研究和治疗试验带来突破。但还需要进行更大规模的研究。
研究人员希望将来可以使用血样而不是脊髓液进行检测。
治疗
帕金森病尚无法治愈,但药物可能有助于控制症状。药物通常效果很好。如果药物不再有效,有些人可能接受手术。医疗护理团队还可能会推荐有氧运动、注重平衡和伸展的物理疗法以及言语治疗。
药物
药物可能有助于改善行走、运动和震颤问题。药物通过增加或替代脑中的多巴胺发挥作用。
帕金森病患者脑中的多巴胺水平较低。但由于多巴胺无法进入脑中,因此无法直接给药。
开始治疗后,您的症状可能会明显改善。随着时间的推移,疗效可能会减弱,但通常药物仍能很好地控制症状。
可能开具的药物包括:
-
卡比多巴-左旋多巴复方制剂(Rytary、Sinemet 等)。 左旋多巴是最有效的帕金森病药物。它是一种天然化学物质,进入脑后会形成多巴胺。左旋多巴与卡比多巴联用,有助于左旋多巴进入脑中,并防止或减轻恶心等副作用。
副作用可能包括恶心和站立时头重脚轻(称为直立性低血压)。较高剂量的左旋多巴可能会导致不自主运动,称为运动障碍。如果出现这种情况,可能需要减少或调整剂量。
左旋多巴的疗效可能会随着时间的推移而减弱。也可能出现波动。这被称为剂末现象。
如果您患有晚期帕金森病,通常最好空腹服用卡比多巴-左旋多巴复方制剂。请遵循医疗护理团队关于最佳服药时间的建议。
- 吸入型左旋多巴(Inbrija)。 当口服药物在白天突然不起作用时,这种药物有助于控制症状。
-
卡比多巴-左旋多巴复方注射液(Duopa)。 这种药物呈凝胶状,通过营养管直接进入小肠。
通过小手术进行插管,插管有一定风险。营养管可能脱落,也可能导致输注部位感染。
卡比多巴-左旋多巴注射液适用于对药物仍有反应但需要更稳定的左旋多巴水平的晚期帕金森病患者。它有助于控制运动障碍以及焦虑和抑郁等其他症状。
-
多巴胺受体激动剂。 与左旋多巴不同,多巴胺受体激动剂不会转化为多巴胺,而是会模拟多巴胺在脑部的作用。
在症状治疗方面,它们不如左旋多巴有效。但它们持续的时间更长,可与左旋多巴一起使用,以改善其疗效。
多巴胺受体激动剂包括:
- 普拉克索(Mirapex ER)。
- 罗替高汀(Neupro),以贴剂形式给药。
- 阿扑吗啡(Apokyn),一种可快速缓解症状的短效多巴胺受体激动剂注射剂。
多巴胺受体激动剂的副作用可能包括头重脚轻、恶心、幻觉和嗜睡。这类药物还可能导致不自主运动和强迫行为,例如性欲亢进、赌博和进食。
如果您在服用这类药物时出现了不寻常的行为,请告知医疗护理团队。
-
单胺氧化酶 B(MAO B)抑制剂。 这些药物包括:
- 司来吉兰(Zelapar)。
- 雷沙吉兰(Azilect)。
- 沙非胺(Xadago)。
MAO B 抑制剂有助于阻断一种被称为单胺氧化酶 B(MAO B)的酶,这种酶会分解脑中的多巴胺。司来吉兰与左旋多巴同时给药,或许能防止左旋多巴产生剂末现象。
MAO B 抑制剂的副作用可能包括头痛、恶心、失眠和意识模糊。
MAO B 抑制剂还可能导致幻觉。如果添加到卡比多巴-左旋多巴复方制剂中,这些药物会增加产生幻觉的风险。
MAO B 抑制剂通常不能与大多数抗抑郁药或某些止痛药同时使用,因为可能会发生严重但罕见的反应。在与 MAO B 抑制剂一起服用任何其他药物之前,请咨询医疗护理专业人员。
-
儿茶酚氧位甲基转移酶(也称为 COMT)抑制剂。 这类药物通过阻断一种分解多巴胺的酶,帮助左旋多巴治疗更持久。这类药物包括:
- 恩他卡朋(Comtan)。
- 奥匹卡朋(Ongentys)。
- 托卡朋(Tasmar)。 很少开具这类药物,因为它有导致严重肝损伤和肝衰竭的风险。
COMT 抑制剂的副作用可能包括不自主运动的风险增加。副作用还可能包括腹泻、恶心或呕吐。
-
抗胆碱能药物。 这些药物已使用多年。由于它们的益处有限且有引起副作用的风险,现在已不再经常使用。这些药物可能有助于控制某些帕金森病患者的重度震颤。这类药物包括:
抗胆碱能药物的副作用可能包括记忆丧失、排尿问题、意识模糊、视力模糊、口腔干燥和便秘。
-
金刚烷胺(Gocovri)。 此药可单独服用,用于短期缓解轻度、早期帕金森病症状。在帕金森病晚期,它主要与卡比多巴-左旋多巴复方制剂一起使用,帮助控制不自主肌肉运动。
金刚烷胺的副作用可能包括皮肤斑驳、思维和记忆问题、脚踝肿胀、幻觉和激越。
- 腺苷受体拮抗剂(A2A 受体拮抗剂)。 这类药物之一是伊曲茶碱(Nourianz)。这类药物有助于防止多巴胺的剂末现象,并可增加多巴胺释放。研究人员还在研究这类药物是否有助于治疗帕金森病的其他症状。
- 哌马色林(Nuplazid)。 这种药物可治疗帕金森病患者可能出现的幻觉和妄想。
手术
脑深部刺激
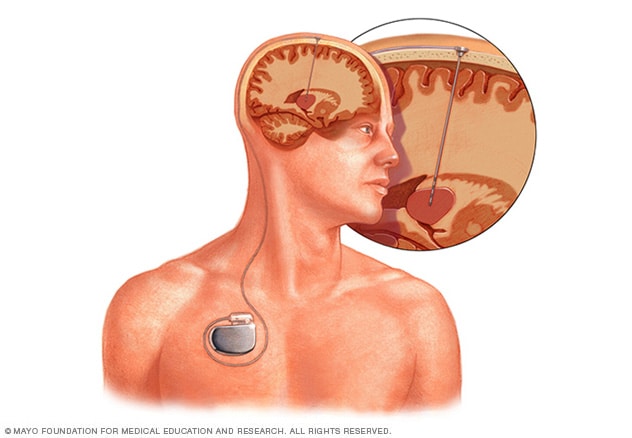
脑深部刺激
脑深部刺激需要将电极置于大脑深处。电极传递的刺激量由放置在胸部皮肤下的一个类似起搏器的装置控制。一根在皮肤下穿行的导线将装置与电极相连。
治疗帕金森病的手术包括脑深部刺激。
脑深部刺激
脑深部刺激(也称为 DBS)涉及将电极置于脑内。电极与插入胸部皮肤下类似起搏器的装置相连。电极与植入胸部锁骨附近的发生器连接。发生器向脑发送电脉冲,可减轻帕金森病症状。
您可能需要复诊以调整设置以获得最佳结果。有些人在使用 DBS 系统时会遇到问题,或者由于刺激而出现并发症。医疗护理团队成员可能需要调整或更换系统的某些部件。
DBS 对于改善重度震颤和控制不自主肌肉运动(称为运动障碍)非常有效。它可以有效控制对左旋多巴的不断变化的反应,也能有效控制无法通过药物变化改善的运动障碍。
脑深部刺激对左旋多巴治疗有效的患者最为有效。虽然 DBS 在帮助缓解症状方面可能有长期益处,但它并不能防止帕金森病加重。研究人员正在研究改进 DBS 的工作效果的方法。
脑深部刺激的副作用可能包括:
- 脑出血。
- 组织损伤或坏死。
- 感染。
- 皮肤断裂。
- 肌肉抽搐。
- 抑郁。
- 言语或视力问题。
先进的治疗方法
MRI 引导聚焦超声(也称为 MRgFUS)检查是一种微创治疗方法,可帮助一些帕金森病患者管理震颤。MRI 将超声引导至震颤开始的脑区域。超声波的温度非常高,会灼烧这些区域。
MRI 引导聚焦超声检查的副作用可能包括:
- 行走和言语问题。
- 新的不自主肌肉运动,也称为运动障碍。
临床试验
探索 Mayo Clinic 的研究 测试新的治疗、干预与检查方法,旨在预防、检测、治疗或控制这种疾病。
生活方式与家庭疗法
一些生活方式改变可能有助于缓解您的帕金森病症状。但某些药物可能会使您的症状加重。询问您的医疗护理团队哪些疗法可以最大程度地缓解症状且副作用最少。
健康饮食
并未证明任何食物可以治疗帕金森病,但有些食物可能有助于缓解症状。例如,食用高纤维食物和饮用足量液体可能有助于预防便秘。
均衡饮食还可提供可能对帕金森病患者有益的营养物质,例如 ω-3 脂肪酸。
运动
运动可能提高您的肌肉力量、行走能力、柔韧性和平衡性,还可能有助于减少抑郁和焦虑。
请医疗护理专业人员推荐一名物理治疗师,帮助您制定运动计划。行走、游泳、园艺、跳舞、水中有氧运动或伸展运动等运动也可能有所帮助。
要改善平衡性和步态,请尝试以下建议:
- 不要移动太快。
- 行走时先放下脚后跟。
- 行走时直视前方,不要往下看。
防止跌倒
以下提示可能会有所帮助:
- 不要着急仓促。
- 不要同时做太多事。
- 使用扶手。
- 使用夜灯。
- 不要使用小块地毯或滚轮椅,把电线收好以免绊倒。
- 学习新的转弯和行走技巧,包括脚跟先着地。此外,走路时站直并目视前方,而不是低头看脚。如果您开始拖着脚走路,请停下来。检查您的姿势,确保站直。
- 如果医疗护理专业人员建议您使用助行器或手杖,请照做。
日常生活活动
这些医疗护理专业人员可以帮助完成日常任务:
- 职业治疗师。 职业治疗师可以向您展示帮助您进行穿衣、浸浴和烹饪等活动的方法。
- 言语治疗师。 言语治疗师或许可以帮助解决吞咽和言语问题。
替代医学
支持性疗法可能有助于缓解帕金森病的一些症状,例如疼痛、疲劳和抑郁。与您的药物治疗相结合,这些疗法可能会改善您的生活质量。包括:
- 按摩。 按摩疗法可减少肌张力,促进放松。这种疗法很少被纳入健康保险范围。
- 太极。 太极是中国传统的运动形式,通过缓慢、流畅的动作来提高灵活性、平衡性和肌肉力量。太极也有助于预防跌倒。有多种太极适合任何年龄或身体状况的人。
- 瑜伽。 瑜伽运动中的轻柔拉伸运动和姿势可以增强灵活性和平衡性。您可以根据自己的身体能力调整大多数姿势。
- 亚历山大矫治技术。 这种技术着重于改善肌肉的姿势和平衡以及更好地思考如何使用肌肉,从而可减少肌张力和疼痛。
- 冥想。 在冥想时,您可以安静地反思,将注意力集中在一个想法或意象上。冥想或许可以减轻压力和痛苦,提高幸福感。
- 放松技巧。 这些做法有助于降低血压和心率并改善肌张力。
- 自我催眠。 这种方法可以帮助您学会在自己的话语或建议的提示下放松。
妥善处理与支持
患任何慢性病的患者的生活可能充满挑战,有时会感到气愤、抑郁或泄气,这都属于正常情况。帕金森病会让人非常沮丧,因为行走、说话甚至吃饭都变得更加困难,并且需要消耗更长的时间。
抑郁在帕金森病患者中较为常见。但抗抑郁药可能有助于缓解抑郁症状。如果您有持续的忧伤或绝望感,请咨询医疗护理团队。
虽然朋友和家人可能是您最好的盟友,但是了解您所经历的事情的人可能特别有帮助。互助组并不一定适合所有人。但是,对于许多帕金森病患者及其家人来说,互助组可以作为获取有关帕金森病的实用信息的好资源。
此外,互助组可提供一个场所,让您找到经历过类似情况并且能够支持您的人。
尝试维持一些日常活动可能会有所帮助。目标是在帕金森病症状开始之前尽可能多做力所能及的事情。关注当下并尝试保持积极的态度。
要了解您所在社区的互助组,请咨询医疗护理团队、帕金森病社工或本地的公共卫生护士。也可以联系帕金森病基金会或美国帕金森病协会。
您及家人也可能从与心理健康专业人员的交谈中受益,例如在与慢性状况患者打交道方面接受过专业培训的心理医生或社工。
准备您的预约
您可能要先去看家庭医疗护理专业人员。然后,您可能会被转诊给在神经系统疾病诊疗领域训练有素的医生(称为神经内科医生)。
由于通常有很多问题需要讨论,所以最好为就诊提前做好准备。以下信息可以帮您做好准备。
您可以做什么
- 写下您目前的所有症状,包括看似与就诊原因无关的症状。
- 写下关键个人信息,包括任何重大压力或近期生活变化。
- 列出您正在使用的所有药物、维生素和补充剂。
- 如果可能,请家人或朋友陪同就诊。有时您可能难以记住就诊时提供给您的所有信息。陪同者可能会记住您遗漏或遗忘的内容。
- 写下要在就诊时咨询的问题。
由于在医护团队处就诊的时间有限,因此提前准备好问题清单可能有助于充分利用就诊时间。关于帕金森病,要咨询的一些基本问题包括:
- 我的症状最有可能是什么原因导致的?
- 是否还有其他可能的原因?
- 我需要做哪些检查? 这些检查需要特殊准备吗?
- 帕金森病通常会如何加重?
- 我最终是否需要长期护理?
- 有哪些治疗方法可供选择?您推荐哪种治疗?
- 预计治疗会有哪些类型的副作用?
- 如果治疗无效或不再有效,是否还有其他选择?
- 我有其他健康状况。如何才能同时管理好这些状况?
- 是否有我可以带回家的手册或其他印刷材料? 您是否可以推荐一些网站?
除了您准备咨询医护团队的问题外,就诊期间您还可以随时提出其他问题。
医生可能做些什么
医疗护理团队可能会询问一些问题。准备好回答这些问题或许可以节省时间,以便更详细地讨论您关心的其他问题。可能会向您询问以下问题:
- 第一次出现症状是在什么时候?
- 您的症状是持续存在,还是时有时无?
- 是否有任何因素似乎会改善您的症状?
- 是否有任何因素似乎会加重您的症状?
Feb. 21, 2025