March 29, 2022
تأثير العلاج بالميغلوستات
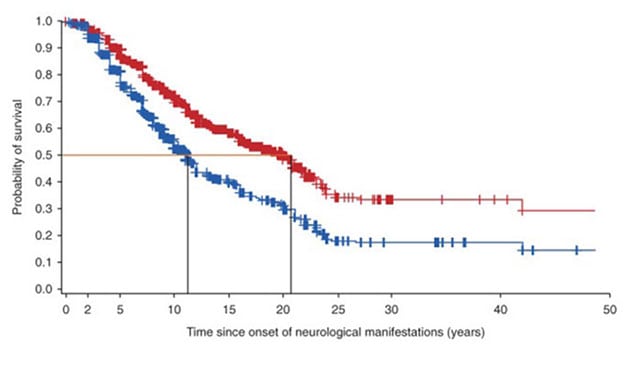
تأثير العلاج بالميغلوستات
يُظهر الجدول منحنيات النجاة للأفراد المصابين بداء نيمان-بيك من النوع ج منذ ظهور العلامات العصبية. يوضح المنحنى الأحمر معدل نجاة الأفراد الذين يتلقون دواء ميغلوستات. بينما يوضح المنحنى الأزرق معدل نجاة الأفراد الذين لا يتلقون دواء ميغلوستات.
Mayo Clinic has expanded its services for children with lysosomal diseases, a group of inherited metabolic disorders with high unmet medical need. Recognized as a center of excellence by the Lysosomal Disease Network, Mayo Clinic offers opportunities for access to new therapies for the treatment of these rare disorders.
"It's a very exciting time. Small-molecule therapies that affect the brain and spinal cord, and in some cases muscles, are becoming available," says Marc C. Patterson, M.D., a pediatric neurologist at Mayo Clinic Children's Center in Rochester, Minnesota. "Gene therapy is also starting to make an impact. Although we're not quite ready for trials in patients, gene therapy has been successfully accomplished in a number of animal models."
Lysosomal diseases can occur at any age but primarily affect children. Individuals experience progressive neurological manifestations that vary greatly among the specific disorders. Neurological symptoms can include developmental delays, movement disorders and seizures. Other symptoms might affect the orthopedic, gastrointestinal, dermatologic, cardiac and ophthalmologic systems. Enzyme replacement therapy — which emerged 30 years ago as a treatment — has been generally effective against somatic symptoms but less effective in treating the neurological aspects.
In addition to leading clinical trials of new therapies, Mayo Clinic is at the forefront of research into the natural history of these disorders. "Predicting the course of disease for individuals is difficult because lysosomal diseases are quite heterogeneous," Dr. Patterson says. "To better measure the effectiveness of therapies, we need to understand that disease course."
Niemann-Pick type C
Mayo Clinic patients have access to two medications — arimoclomol and miglustat — for the treatment of Niemann-Pick type C (NPC), a neurodegenerative disease that can present from the perinatal period into adulthood. Earlier onset is associated with greater severity and more rapid progression.
Arimoclomol is a small-molecule heat shock therapy. As described in the November 2021 issue of the Journal of Inherited Metabolic Disease, Mayo Clinic co-led a multinational clinical trial of the drug. "The results strongly supported the role of arimoclomol in slowing the progression of NPC," Dr. Patterson says.
Miglustat, a small-molecule enzyme inhibitor, has been approved for the treatment of NPC in the European Union and several other countries. An observational study of the largest cohort to date of individuals with NPC — published in the September 2020 issue of the Journal of Inherited Metabolic Disease — found that the drug was associated with a significant reduction in the risk of mortality.
"The data suggest that miglustat increases the life span of patients with NPC by approximately 10 years from the onset of neurological manifestations and by five years from the diagnosis of the disease," Dr. Patterson says.
He notes that a subgroup of individuals in the 2021 study who received miglustat in addition to arimoclomol had even better outcomes than participants who received arimoclomol alone. "That suggests that a combination of these treatments might be more effective," Dr. Patterson says. "Both of the drugs were well tolerated, which is encouraging."
Infantile metachromatic leukodystrophy
Mayo Clinic has also seen encouraging results from a clinical trial of a recombinant human enzyme for the treatment of infantile metachromatic leukodystrophy. Like NPC, metachromatic leukodystrophy can occur at any age. Onset during infancy is more common and severe than later onset.
"Untreated, this disorder is usually rapidly fatal in infants. In older children, it's disabling and substantially shortens life spans," Dr. Patterson says. "Children lose the ability to walk. The disorder affects not only the central white matter but also the peripheral nerves, so children lose their reflexes. They have visual loss as well as cognitive regression."
The clinical trial involved intrathecal injections of the recombinant human enzyme known as SHP611. "The data suggest that disease progression can be slowed or even stabilized, if the disease is recognized early and treatment is started," Dr. Patterson says. "That is a really important advance."
Disease registry
Mayo Clinic is committed to further understanding the natural history of lysosomal diseases. "Although we typically are able to predict the sequence of events for an individual's disease progression, predicting the timing is difficult," Dr. Patterson says.
A comprehensive disease registry is an important part of this work. Dr. Patterson chairs the Scientific Advisory Committee of the International Niemann-Pick Disease Registry, a database encompassing medical records and patient-supplied data.
Lysosomal disease registries are typically created by companies sponsoring clinical trials. "Unfortunately, the data tend to be lost once a trial is done," Dr. Patterson says. "We hope this new registry will be a model for future collaboration of patients, clinicians, laboratory researchers and industry, and a resource for moving the field forward."
Another key approach is facilitating earlier diagnosis. "By the time patients have neurological symptoms, a substantial proportion of cells in the nervous system are likely to be malfunctioning or lost. The greater the burden of disease, the less likely we are able to reverse these manifestations," Dr. Patterson says.
To that end, Mayo Clinic is using informatics to refine newborn screening for certain lysosomal diseases. Several states now include those diseases in their newborn screening.
"Lysosomal disorders are really awful diseases. But we're making progress," Dr. Patterson says. "The ideal would be to identify these diseases very early and then deliver effective therapy, so these patients never become symptomatic."
For more information
Mengel E, et al. Efficacy and safety of arimoclomol in Niemann-Pick disease type C: Results from a double-blind, randomised, placebo-controlled, multinational phase 2/3 trial of a novel treatment. Journal of Inherited Metabolic Disease. 2021;44:1463.
Patterson MC, et al. Long-term survival outcomes of patients with Niemann-Pick disease type C receiving miglustat treatment: A large retrospective observational study. Journal of Inherited Metabolic Disease. 2020;43:1060.
International Niemann-Pick Disease Registry.
Refer a patient to Mayo Clinic.