Oct. 26, 2021
Oncogenic osteomalacia, also referred to as tumor-induced osteomalacia (TIO), is a rare disorder characterized by urinary phosphate wasting due to an increased production of fibroblast growth factor 23 (FGF23) from an underlying mesenchymal tumor. This wasting results in a significant reduction in serum phosphate and leads to bone pain, muscle weakness and skeletal deformities, as well as recurrent fractures. These symptoms cause significant disability and morbidity in patients with this disease.
The main challenges with patients with TIO are the identification of the disease and the localization of the FGF23-producing tumor.
"It is very common for TIO to remain undiagnosed for several months or years, largely owing to the nonspecificity of the presenting symptoms as well as limited awareness of the disease," reports Jad Sfeir, M.D., M.S., Endocrinology, Diabetes, Metabolism, and Nutrition, at Mayo Clinic in Rochester, Minnesota.
On average, TIO is diagnosed 2.9 years after the onset of symptoms. The most widely available assays measuring serum or plasma FGF23 detect the carboxy-terminal fragment. Recently, an assay measuring the biologically active intact molecule has been developed. "The intact FGF23 assay is available at Mayo Clinic Laboratories, and we are collecting samples from patients with TIO to establish its clinical performance," confirms Alicia Algeciras-Schimnich, Ph.D., Laboratory Medicine and Pathology, at Mayo Clinic in Minnesota.
"There is also a big gap in time between the diagnosis of TIO and the identification of the underlying tumor of an average of 5.4 years, with some reports of patients waiting as long as 40 years before the tumor was resected," adds Matthew T. Drake, M.D., Ph.D., Endocrinology, Diabetes, Metabolism, and Nutrition, at Mayo Clinic in Minnesota. Resection of the tumor is usually curative as it removes the source of excess FGF23, and thus timely localization of the tumor is essential.
الكتلة المتكلسة على يمين الحفرة القحفية الوسطى
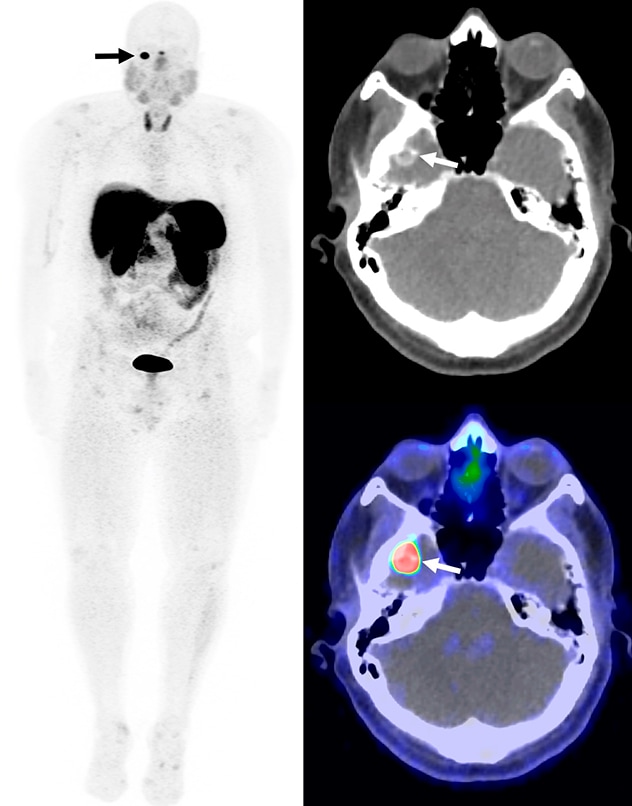
الكتلة المتكلسة على يمين الحفرة القحفية الوسطى
تظهر كتلة متكلسة كثيفة الامتصاص في الحفرة القحفية في تصوير الكثافة القصوى للجسم بالكامل، والتصوير المقطعي المحوسب، والتصوير المقطعي بالإصدار البوزيتروني/التصوير المقطعي المحوسب المدمجان المستخدم فيها الغاليوم 68 (68Ga)-دوتاتات التصوير المقطعي بالإصدار البوزيتروني/التصوير المقطعي المحوسب (الأسهُم). وقد استُئصلت الكتلة، وتم التأكد من أنها ورم ناتج عن تليُّن عظمي متوسطي.
DOTATATE PET CT and other Gallium 68 (68Ga)-conjugated somatostatin peptide analogues have been reported to localize FGF23-producing mesenchymal tumors. This technology has been validated for the use in patients with neuro-endocrine tumors but not in TIO. "We have initiated a clinical trial to investigate the use of 68Ga-DOTATATE PET CT in patients with TIO, which could become a game changer in the timely management of these patients," states Stephen M. Broski, M.D., Diagnostic Radiology, at Mayo Clinic in Minnesota.
An FGF23 monoclonal antibody (burosumab) was recently approved by the U.S. Food and Drug Administration for use in patients in whom surgical tumor resection is not possible. "Burosumab showed biochemical and clinical benefits in phase 2 clinical trials. However, since nonsurgical patients are likely to require long-term medical management, data on longer duration and larger scale use of burosumab in patients with TIO would help identify clinical recommendations for its use," concludes Dr. Sfeir.
Clinical trials at Mayo Clinic
Current research related to FGF23-mediated oncogenic osteomalacia and FGF23 assays includes:
Ga-DOTATATE PET CT for localization of phosphaturic mesenchymal tumors in patients with tumor-induced osteomalacia
Mayo Clinic investigators are studying the use of 68Ga-DOTATATE PET CT to localize the underlying mesenchymal tumor in patients with FGF23-mediated oncogenic osteomalacia.
Interested patients or referring providers can contact Jad Sfeir, M.D., M.S., and view additional information and study contacts at Ga-DOTATATE PET CT for Localization of Phosphaturic Mesenchymal Tumors in Patients With Tumor-induced Osteomalacia.
A study to evaluate intact FGF23 performance in patients with tumor-induced osteomalacia (TIO) and X-linked hypophosphatemia (XLH)
Investigators from Laboratory Medicine and Pathology at Mayo Clinic in Minnesota aim to establish the clinical performance of the intact FGF23 assays.
Interested patients or referring providers can contact Stephanie S. Hafner, Laboratory Medicine and Pathology, at Mayo Clinic in Minnesota and view additional information and study contacts at A Study to Evaluate Intact FGF23 Performance in Patients With Tumor-Induced Osteomalacia (TIO) and X-linked Hypophosphatemia (XLH).
For more information
Mayo Clinic Laboratories. Mayo Clinic.