Oct. 02, 2020
Mayo Clinic is committed to providing the least invasive care that will be effective for patients with refractory epilepsy. Treatment options include a range of neurostimulation and ablation techniques, including radiofrequency ablation, as well as laser corpus callosotomy.
"Our goal is to be as minimally invasive as possible, so we can treat the epilepsy while preserving function," says Kai J. Miller, M.D., Ph.D., a neurosurgeon at Mayo Clinic in Rochester, Minnesota. "As technology has advanced, we are now able to ablate sources of seizure with focal laser or electrocautery and avoid an open resection unless absolutely necessary."
Dr. Miller's clinical focus is pediatric neurosurgery, with an emphasis on epilepsy. In conjunction with Dora Hermes, Ph.D., a neurophysiologist at Mayo's campus in Minnesota, Dr. Miller also researches brain signaling and the effects of neurostimulation in adults and children. Based on electrophysiological recordings made intraoperatively and in the epilepsy monitoring unit, the neurostimulation studies help develop implantation techniques that provide more anatomically characterized recordings.
Mayo Clinic's efforts will aid the creation of the next generation of neurostimulation therapies, aimed not only at placing electrodes more precisely but also at disrupting the networks that propagate epileptic activity among brain regions.
"We don't yet understand how stimulation affects not just the area we're stimulating but also other brain areas connected to it," Dr. Miller says. "Decoding brain activity will help us localize seizure origin for better placement of electrodes. It can also open up new mechanisms. We'd like to be able to implant devices that record brain activity continuously, decode it and stimulate the brain in such a way that, over time, an epilepsy network becomes less effective at producing seizures. We will help patients to actually recover. That's the home run."
Achieving concordance
Definitive epilepsy therapy is facilitated by concordance between a patient's imaging and electrophysiological testing. Much of the research done by Dr. Miller and Dr. Hermes involves looking for new ways to achieve that concordance.
"We want to understand the underlying neuronal activity that drives a functional MRI signal," Dr. Hermes says. "That type of biomarker will help us localize and treat disease." Mayo Clinic's 7-tesla MRI, which helps to define structures that might not be visible at lower resolution, is a key resource. Computational models and simulations of neuronal population activities are also important components of this work. "Since we can never measure every single neuron, we have to make a model of the underlying neuronal activity in order to understand the signaling and to predict brain activity," Dr. Hermes says.
As described in the Nov. 8, 2019, edition of eLife, her research team developed a model that predicts from visual input the type of signals produced in the visual cortex of an individual without epilepsy. "We are now trying to translate those findings into predictions of atypical brain activity," Dr. Hermes says.
The researchers have also found that the same visual input that induces narrow band gamma oscillations around 50 hertz can also induce seizures in patients with pattern-sensitive epilepsy. "We think that the same circuitry involved in these oscillations might be changed in patients with photosensitive epilepsy," Dr. Hermes says. "Ultimately, this information can help us influence these networks to reduce brain excitability in patients."
用于研究单次癫痫发作网络中不同节点之间癫痫相关相互作用的系统
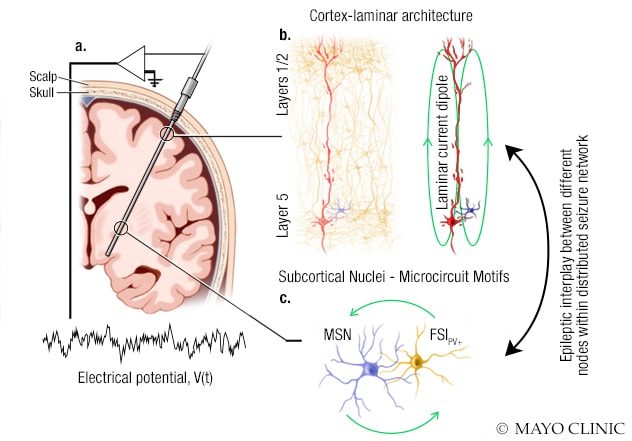
用于研究单次癫痫发作网络中不同节点之间癫痫相关相互作用的系统
此图所示为妙佑医疗国际的研究人员用于研究单次癫痫发作网络中不同节点之间的癫痫相关相互作用的系统。A. 立体定向脑电探头已被植入脑内以监测电活动。电位通过此探头上的几个电极加以记录。B. 有些电极会记录具有层状结构的皮层回路产生的场电位。皮层场电位由大锥体神经元产生的电流偶极子强力驱动。C. 其他电极会记录皮层下区域神经元回路、中等棘状神经元 (MSN) 和小清蛋白阳性 (PV+) 快闪中间神经元 (FSI) 产生的场电位。
Understanding the interactions among nodes in a seizure network can potentially facilitate neurostimulation treatments that modify a patient's disease, ultimately obviating the need for an implanted device. "That's not something we'll accomplish in five years — but maybe in 10 years," Dr. Miller says.
As the practices of neurostimulator placement and stereotactic laser ablation evolve, optimal targeting will require standardized outcome measures that compare electrode lead or laser source with post-procedural changes in seizure frequency. Targeting is currently prescribed for general anatomical structures. Dr. Miller led a team that developed a stereotactic coordinate system based on landmarks of the mesial temporal lobe. As described in the January 2018 edition of Journal of Neurosurgery, the researchers created an open-source software package to test the system.
The overarching goal of Mayo Clinic's neurostimulation research is to translate innovations into patient care. "The collaborative structure of Mayo Clinic means that physicians see the potential impact of research for patients," Dr. Hermes says. "At Mayo, there is a route for innovations to become standard clinical practice.
For more information
Hermes D, et al. An image-computable model for the stimulus selectivity of gamma oscillations. ELife. 2019;8:e47035.
Miller KJ, et al. A novel mesial temporal stereotactic coordinate system. Journal of Neurosurgery. 2018;130:67.