Sept. 14, 2018
A 68-year-old man was referred to the endocrine clinic for evaluation of abnormal thyroid function tests. His medical history was notable for metastatic malignant melanoma, initially located at the right thigh, for which he underwent wide local excision and extensive lymph node dissection three years earlier. The following year, he developed local recurrence and new pelvic lymphadenopathy, for which he underwent resection followed by radiation therapy. Due to disease progression (BRAF wild type), the patient was initiated on ipilimumab, an immune checkpoint inhibitor targeting cytotoxic T-lymphocyte associated protein 4 (CTLA-4). The patient received four cycles of ipilimumab — 3 mg/kg/day every three weeks.
His disease remained refractory, and he was then initiated on a second immune checkpoint inhibitor targeting programmed death receptor-1 (PD-1), pembrolizumab 2 mg/kg/day every three weeks. As part of surveillance for immune-related adverse events (irAEs), thyroid function tests were checked: TSH = 0.01 mIU/L (normal, 0.3-4.2 mIU/L) and free T4 = 2.2 ng/dL (normal, 0.9-1.7 ng/dL). The patient had moderate fatigue; he denied tremors or palpitations. His family history was significant for a sister with primary hypothyroidism.
Increased thyroid vascularity

Increased thyroid vascularity
Thyroid ultrasound showing diffuse heterogeneity of the thyroid gland.
Increased thyroid vascularity

Increased thyroid vascularity
Doppler ultrasound demonstrating increased thyroid vascularity.
Diffusely decreased uptake, consistent with thyroiditis
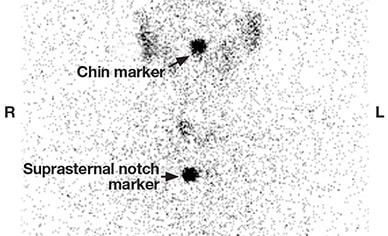
Diffusely decreased uptake, consistent with thyroiditis
Thyroid uptake and scan showing diffusely decreased uptake, consistent with thyroiditis. The 23-hour radioactive iodine uptake was 0.6 percent (normal 24-hour uptake = 8-29 percent).
Neck at thyroid gland level pre- and post-surgery

Neck at thyroid gland level pre- and post-surgery
18F-fluorodeoxyglucose positron emission tomography scan of the neck at the level of the thyroid gland. A. Prior to pembrolizumab therapy. B. Six months after pembrolizumab initiation.
Further evaluation in the endocrine clinic demonstrated a normal erythrocyte sedimentation rate and normal levels of TRAB and TPO antibodies. Sonographic examination of the neck demonstrated diffuse heterogeneity of the thyroid gland suggestive of thyroiditis and low to absent iodine uptake on thyroid scan confirmed thyroiditis. Restaging imaging for his melanoma with 18F-fluorodeoxyglucose positron emission tomography demonstrated new bilateral thyroid uptake more pronounced on the previously negative left thyroid lobe.
Repeat thyroid function tests three weeks later showed progression to overt hypothyroidism: TSH = 13.1 mIU/L, total T3 = 69 ng/dL (normal, 80-200 ng/dL), and free T4 = 0.9 ng/dL. The patient was initiated on levothyroxine replacement therapy and treatment with pembrolizumab was continued.
Immune modulators
The immune checkpoint inhibitors pembrolizumab, ipilimumab and nivolumab represent a novel class of immune-directed anti-neoplastic therapies with significant anti-tumor efficacy. These fully humanized, monoclonal antibodies block negative regulatory receptors (for example, CTLA-4 or PD-1) on T cells, resulting in a de-repression or reactivation of cytotoxic T cell function or both.
Clinically, this translates into durable tumor regression in patients with melanoma, lung cancer, renal cell carcinoma or Hodgkin's lymphoma. The list of Food and Drug Administration-approved malignancies for treatment with these immune therapies continues to expand as clinical trials in a wide variety of tumor types are demonstrating tumor efficacy.
As expected, however, immune modulators have introduced unique immune-based toxicities. Thyroid abnormalities and hypophysitis are the two most common endocrine irAEs, although primary adrenal insufficiency and type 1 diabetes mellitus have also been reported.
The endocrine clinic recently reviewed thyroid abnormalities among patients with cancer receiving pembrolizumab. Thyroid dysfunction occurred in approximately 15 percent of patients and most often presented as an acute painless thyroiditis or overt hypothyroidism. The median onset of thyroid abnormalities was within the first two to four weeks following treatment initiation with either ipilimumab or pembrolizumab.
In accordance with a study published in The Journal of Clinical Endocrinology & Metabolism in 2016, thyrotoxicosis is usually transient with either regain of normal thyroid function or evolution to hypothyroidism. Thyroid autoantibodies are variably elevated in some patients, but most often normal. Rare cases of Graves' disease and ophthalmopathy have been reported.
In current clinical trials published in Current Opinion in Oncology in 2016 and Endocrine-Related Cancer in 2014, thyroid dysfunction is reported in 1 to 15 percent of patients treated with pembrolizumab and 0 to 4 percent of patients treated with ipilimumab. Occasionally, a low TSH may indicate the presence of a central process consistent with immune therapy-induced hypophysitis. Hypophysitis is uncommon with anti-PD-1 therapy and more frequent with anti-CTLA-4 therapy.
Management of immune modulator-induced acute thyroiditis is similar to other etiologies (for example, symptomatic-based therapy with beta-adrenergic blockers, if indicated). Levothyroxine replacement is appropriate for overt hypothyroidism, although some patients may recover thyroid function. The endocrine clinic has commonly observed thyroid hormone abnormalities coincident with new, diffuse increased fludeoxyglucose uptake in the thyroid that resolves on follow-up imaging, suggesting an underlying immune-mediated destructive inflammatory process rather than metastatic tumor spread.
Our patient developed frank hypothyroidism and was initiated on levothyroxine replacement therapy while continuing immunotherapy. The underlying mechanism of immune-mediated thyroid dysfunction and the role of thyroid autoantibodies in this anti-thyroid immune response remain unknown.
Conclusions
The indications for immune checkpoint inhibitors for patients with advanced cancer have greatly expanded within the last five years. Endocrinologists and medical oncology teams should be familiar with their unique endocrine-related irAEs in order to minimize morbidity and enable appropriate management, as indicated by research published in Melanoma Research in 2016. Thyroiditis may need supportive care, with monitoring for development of hypothyroidism.
Thyroid hormone replacement therapy is often required for immune therapy-induced hypothyroidism, though thyroid recovery may also occur. Importantly, serial measurements of thyroid function tests are indicated, especially during the first weeks of pembrolizumab therapy.
Further studies with histopathological correlates are required for a better understanding of the underlying immune cell subtypes that mediate these irAEs. Importantly, there are now ongoing or soon to open clinical trials attempting to harness this anti-thyroid immune response with immune checkpoint inhibitors for patients with advanced, radioiodine refractory thyroid cancer, as well as for anaplastic thyroid cancers.
For more information
De Filette J, et al. Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. The Journal of Clinical Endocrinology & Metabolism. 2016;101:4431.
Torino F, et al. Endocrinological side-effects of immune checkpoint inhibitors. Current Opinion in Oncology. 2016;28:278.
Ryder M, et al. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocrine-Related Cancer. 2014;21:371.
Kottschade L, et al. A multidisciplinary approach to toxicity management of modern immune checkpoint inhibitors in cancer therapy. Melanoma Research. 2016;26:469.