Feb. 24, 2024
Diabetes is recognized as the most expensive chronic disease globally, and it is a challenge to manage. Long-term failure to achieve consistent glucose control can increase an individual's risk of cardiovascular disease, stroke, cancer, kidney disease and other undesirable outcomes.
Endoscopic re-cellularization via electroporation therapy (ReCET), also known as endoscopic duodenal mucosal regeneration, is a novel endoscopic procedure and device developed by Mayo Clinic researchers and collaborators in Minnesota to help individuals with difficult-to-control type 2 diabetes.
In this Q&A, Andrew C. Storm, M.D., a gastroenterologist and director of endoscopy at Mayo Clinic in Minnesota, discusses this approach to treating type 2 diabetes and the ongoing research efforts to evaluate the safety and efficacy of ReCET.
Can you describe what factors have led to the development of ReCET?
Over the years, the tools and technology and interventions designed to help manage diabetes have improved. However, the increasing prevalence of this disease worldwide underscores the need for novel therapeutic approaches. In some ways, we are making great progress in treating diabetes; but in other ways, we're failing systemically. We need novel approaches that go beyond traditional medications, delivery devices, and management protocols or interventions.
Can you describe the goal of the ReCET procedure and how it works?
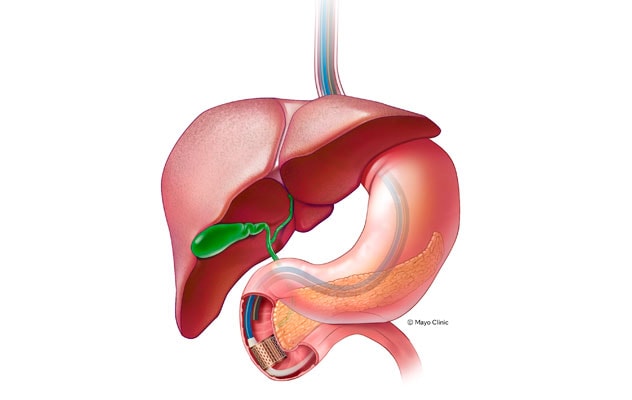 Endoscopic re-cellularization via electroporation therapy (ReCET)
Endoscopic re-cellularization via electroporation therapy (ReCET)
ReCET is nonthermal procedure that involves delivering a pulsed electric field (PEF) to facilitate the re-cellularization of the duodenum with regenerated metabolically active cells. Using an endoscope to position the ReCET catheter, the endoscopist delivers PEF to the duodenum. The goal of this procedure is to help individuals achieve better control of blood glucose levels by correcting how the duodenum functions.
The ReCET procedure was invented, designed and prototyped in Minnesota, with the goal of disrupting the treatment of type 2 diabetes for patients who want to try an approach that doesn't involve more needles and medications to control their disease. This nonthermal procedure involves delivering a pulsed electric field (PEF) to facilitate the re-cellularization of the duodenum with regenerated metabolically active cells. Using an endoscope to position the ReCET catheter, the endoscopist delivers PEF to the duodenum. The goal of this procedure is to help individuals achieve better control of blood glucose levels by correcting how the duodenum functions. One of the key benefits of this approach is that it is a single outpatient procedure that doesn't require daily doses of pharmacotherapy.
What can you tell us about clinical safety and efficacy testing performed to date?
The REGENT-1-US trial is a prospective multicenter trial designed to assess the safety, feasibility and efficacy of the ReCET approach. The trial is being conducted at Mayo Clinic and another site in Minnesota and at the University of Southern California. Study participants are individuals between ages 22 and 65 with type 2 diabetes that is difficult to control who meet the following eligibility criteria: taking 2 to 3 noninsulin glucose-lowering medications, HbA1c of 7.5% to 10% and body mass index of 24 to 40 kg/m2. Study participants are followed for 12 months and undergo endoscopy at four weeks.
At the 2023 American Society for Metabolic and Bariatric Surgery Annual Meeting, the ReCET clinical trial team presented an abstract of preliminary findings from the REGENT-1-US trial. The findings presented in the abstract include data from 10 participants and demonstrated a 100% success rate in the procedure, with no serious adverse events reported. Most notably, four of the five participants who completed the 24-week follow-up achieved an HbA1c level of ≤ 7.0%. Mean weight loss among participants was 5.0% ± 3.8% at 24 weeks.
What are the next steps related to this novel approach, and how might ReCET impact the care burden experienced by individuals with type 2 diabetes in the future?
Although ReCET has not yet received Food and Drug Administration (FDA) approval, the research team is hopeful that the approach will undergo broader use and study after approval. This novel procedure could potentially transform the treatment landscape for type 2 diabetes, offering a more efficient and less burdensome alternative to current pharmacotherapies for some individuals. The procedure's potential to reduce the daily burden associated with diabetes management, coupled with the significant preliminary results of the REGENT-1-US trial, positions ReCET as a promising alternative in the diabetes care arsenal. As we await the conclusion of the trial and FDA approval, we are optimistic about the potential impact of this novel procedure on the lives of people coping with this challenging chronic disease.
For more information
Endogenex, Inc. Safety and Feasibility of Novel Therapy for Duodenal Mucosal Regeneration for Type II Diabetes (REGENT-1-US). ClinicalTrials.gov.
Abu Dayyeh BK, et al. Endoscopic re-cellularization via electroporation therapy (ReCET) for improving glycemic control in individuals with type 2 diabetes — Interim report of prospective feasibility trial. Presentation at: American Society for Metabolic and Bariatric Surgery Annual Meeting; 2023; Las Vegas.
Refer a patient to Mayo Clinic.