Oct. 20, 2022
Endoscopic sleeve gastroplasty
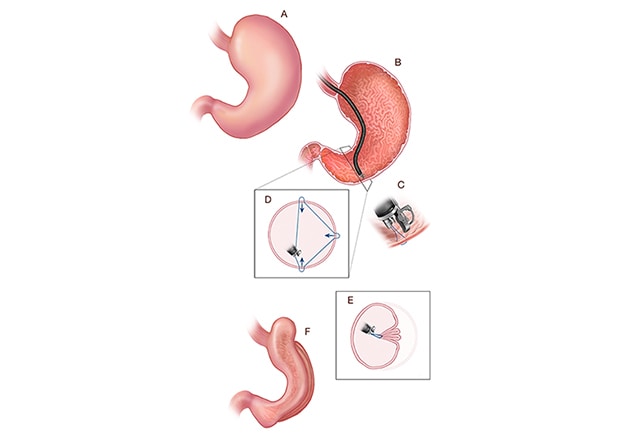
Endoscopic sleeve gastroplasty
A. Stomach before endoscopic sleeve gastroplasty (ESG). B-E. The steps involved in creating the ESG using an endoscope and a full-thickness endoscopic suturing device. F. Final configuration of the stomach after ESG.
Endoscopic sleeve gastroplasty (ESG) is a reversible and minimally invasive form of therapy for obesity that is widely used throughout the world. ESG is an endoscopic anatomy-preserving weight-loss procedure that relies on a full-thickness endoscopic suturing device to reduce stomach volume by 80% through the creation of a restrictive endoscopic sleeve. This is accomplished through a series of endolumenally placed, full-thickness sutures in the gastric wall, extending from the antrum to the gastroesophageal junction.
In the Multi-center ESG Randomized Interventional Trial (MERIT-Trial), investigators from Mayo Clinic in Rochester, Minnesota, and eight other centers in the United States examined the efficacy and safety of ESG in patients with class 1 or class 2 obesity who failed to achieve and maintain weight loss with a nonsurgical program. The results of the MERIT-Trial were published in The Lancet in 2022, with Mayo Clinic gastroenterologist Barham K. Abu Dayyeh, M.D., M.P.H., serving as lead author. Dr. Abu Dayyeh is the associate research chair for device and procedural innovation in Internal Medicine, and the director of Advanced Endoscopy at Mayo Clinic in Rochester, Minnesota.
Methods
The MERIT-Trial involved nine sites in the United States and enrolled 209 individuals ages 21 to 65 years with class 1 or class 2 obesity (body mass index between 30 and 40 kg/m²). Study participants agreed to comply with lifelong dietary restrictions and were randomly assigned to ESG with lifestyle modifications (ESG group, n = 85), or to lifestyle modifications alone (control group, n = 124). Lifestyle modifications that both groups underwent included a low-calorie diet and physical activity.
The researchers followed participants in the primary ESG group for 104 weeks. At 52 weeks, the researchers calculated the percentage of excess weight loss (EWL), the study's primary endpoint. Participants in the control group who completed the 52-week visit and did not reach the target primary weight-loss goal (≥ 25% EWL) were offered ESG and were followed for an additional 52 weeks after crossover. The researchers also noted any change in metabolic comorbidities between the groups, the study's secondary endpoints.
Results
At 52 weeks, the researchers noted the following findings:
- The mean percentage of EWL was 49.2% for the ESG group and 3.2% for the control group (p < 0.0001).
- The mean percentage of total body weight loss was 13.6% for the ESG group and 0.8% for the control group (p < 0.0001). In the ESG group, 77% of participants reached 25% or more of EWL at 52 weeks, compared with 12% in the control group (p < 0.0001).
- In the ESG group, 80% of participants had an improvement in one or more metabolic comorbidities, compared with 45% in the control group.
At 104 weeks, 68% of participants in the ESG group maintained 25% or more of EWL. ESG-related serious adverse events occurred in 2% of participants, with no related deaths or need for intensive care or surgery.
"Overall, the MERIT-Trial results demonstrate that ESG, combined with lifestyle modifications, is a safe intervention that can help patients with class 1 or class 2 obesity achieve and maintain significant weight loss, as well as important improvements in metabolic comorbidities," summarizes Dr. Abu Dayyeh. "Our findings also demonstrate that ESG is scalable and can be offered in outpatient endoscopy practices by surgeons or gastroenterologists, with an excellent safety profile."
For more information
Abu Dayyeh BK, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): A prospective, multicentre, randomised trial. The Lancet. 2022;400:441.
Refer a patient to Mayo Clinic.