April 13, 2018
Pancreatic ductal adenocarcinoma

Pancreatic ductal adenocarcinoma
The sketch and endoscopic ultrasound image demonstrate the typical appearance of a pancreatic ductal adenocarcinoma.
"Tumoremia" is a term that refers to the presence of cellular and nuclear material from tumors in the bloodstream. These cells and nuclear material can be detected in the blood as circulating tumor cells (CTCs) or cell-free DNA (cfDNA). Although endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is thought to be safe, some experts question whether tumor manipulation during biopsy causes tumors to shed these types of cells into the bloodstream. Most patients who undergo resection for pancreatic adenocarcinoma (PDAC) develop local recurrence, distant metastasis or both, but it is unclear whether procedures such as EUS-FNA cause tumoremia or contribute to formation of metastases.
To assess whether EUS-FNA of primary tumors is associated with markers of tumoremia, Mayo Clinic researchers conducted a prospective study of blood samples from patients with PDAC. These study results were published in a recent issue of Clinical Gastroenterology and Hepatology.
Study goals and methods
Mayo researchers had two primary aims:
- To determine the incidence of FNA-induced tumoremia
- To determine the feasibility and safety of serial portal vein (PV) and hepatic vein (HV) blood collections
The research team collected and studied peripheral blood samples from 104 individuals. Study participants included 35 individuals with PDAC (tested before and after EUS-FNA of primary tumors) and 69 healthy individuals (control group). Researchers measured plasma concentrations of cfDNA and analyzed cfDNA and primary tumor samples to detect the presence of activating mutations in the KRAS gene. This gene makes a protein called KRAS, which is involved in cell signaling pathways that control cell growth, cell maturation and cell death.
Droplet digital PCR (ddPCR) analysis of cell-free DNA (cfDNA) shows sample positive for a mutation in codon 12/13 in KRAS gene
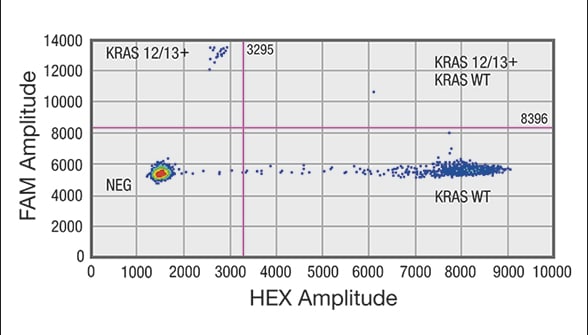
Droplet digital PCR (ddPCR) analysis of cell-free DNA (cfDNA) shows sample positive for a mutation in codon 12/13 in KRAS gene
Distribution of droplets is visualized using a heat map. Droplet thresholds are shown as pink lines. Signal detected in the channel represents DNA positive for a KRAS 12/13 mutation. Signal detected in HEX channel represents DNA positive for KRAS wild-type (WT) amplification in which cfDNA mutants were nondetectable. A. Sample positive for a mutation in codon 12/13 in KRAS gene. Distribution of negative droplets (NEG), droplets positive for mutation in KRAS codon 12/13 (KRAS 12/13+) and droplets positive for KRAS WT.
The presence of tumoremia was defined as follows: any increase in cfDNA of twofold or more, and/or the presence of mutant KRAS in samples collected after FNA from individuals whose blood samples did not contain detectable mutant KRAS before FNA.
Results
Droplet digital PCR (ddPCR) analysis of cell-free DNA (cfDNA) shows sample negative for mutation in codon 12/13 of KRAS gene
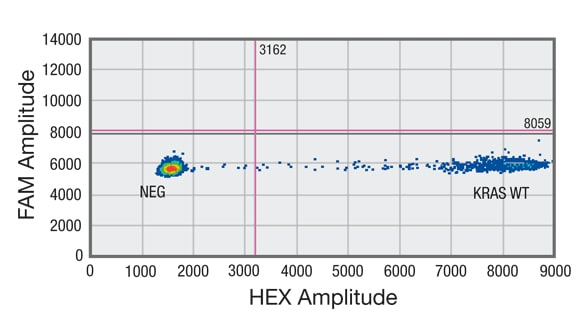
Droplet digital PCR (ddPCR) analysis of cell-free DNA (cfDNA) shows sample negative for mutation in codon 12/13 of KRAS gene
Distribution of droplets is visualized using a heat map. Droplet thresholds are shown as pink lines. Signal detected in the channel represents DNA positive for a KRAS 12/13 mutation. Signal detected in HEX channel represents DNA positive for KRAS wild-type (WT) amplification in which cfDNA mutants were nondetectable. B. Sample negative for mutation in codon 12/13 of KRAS gene but showing amplification of KRAS WT.
Droplet digital PCR (ddPCR) analysis of cell-free DNA (cfDNA) shows sample without DNA showing no positive droplets in either FAM or HEX channel
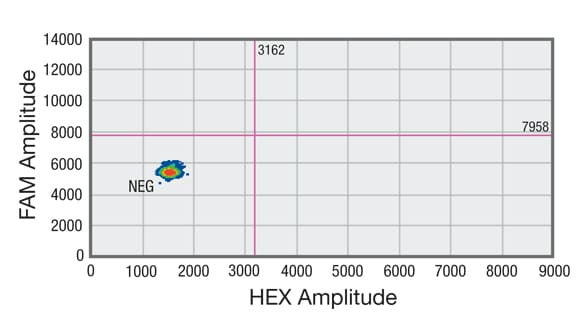
Droplet digital PCR (ddPCR) analysis of cell-free DNA (cfDNA) shows sample without DNA showing no positive droplets in either FAM or HEX channel
Distribution of droplets is visualized using a heat map. Droplet thresholds are shown as pink lines. Signal detected in the channel represents DNA positive for a KRAS 12/13 mutation. Signal detected in HEX channel represents DNA positive for KRAS wild-type (WT) amplification in which cfDNA mutants were nondetectable. C. Sample without DNA showing no positive droplets in either FAM or HEX channel.
- Peripheral blood concentrations of cfDNA were 1,200 ng/ml (500 to 3,300 ng/ml) before FNA and 1,400 ng/ml (900 to 4,000 ng/ml) after FNA (p = 0.391).
- Tumoremia was detected in 10 out of 35 participants with PDAC (28.6 percent); seven participants with PDAC had a ≥ twofold increase in cfDNA concentration (20.6 percent); and three participants with PDAC had circulating tumor DNA with KRAS mutations after FNA that were not detected before FNA (8.8 percent).
- New distant metastases were detected in 1.3 ± 0.82 participants with tumoremia and in 0.64 ± 0.81 participants without (p = 0.0375).
- Overall mortality did not differ significantly between participants with tumoremia and without tumoremia: 10 out of 10 deaths, or 100 percent versus 19 out of 25 deaths, or 76 percent.
- Overall survival times of deceased participants did not differ significantly between participants with and without tumoremia: 13.3 months, with a range of 5.8 to 14.9 months, versus 11.1 months, with a range of 5.5 to 14.5 months. However, at a mean of 23.9 months after EUS-FNA, six participants without tumoremia were alive (range of 19.9 to 25 months after EUS-FNA) and zero participants with tumoremia were alive.
Conclusions
"Our data suggest that primary tumor FNA may release nuclear material or cells into the circulation," explains Michael J. Levy, M.D., a gastroenterologist specializing in interventional endoscopy and pancreatobiliary disorders at Mayo Clinic's campus in Minnesota and the study's lead author. "In patients with PDAC, EUS-FNA is associated with increased plasma concentration of cfDNA and increased detection of mutant KRAS after the procedure, both of which are markers of tumoremia and possible new distant metastasis."
Dr. Levy cautions that these findings are preliminary and do not definitively indicate a heightened risk of FNA-induced tumoremia or a need to modify current practice. Although levels of cfDNA and activating mutations in KRAS are logical markers of tumoremia, Dr. Levy notes that they may not be the ideal biomarkers for this process. "Further studies are needed to confirm our study results, to assess the utility of testing for quantitative and qualitative cfDNA and ctDNA and central blood sampling, and to measure any impact on patient outcomes," explains Dr. Levy.
For more information
Levy, MJ, et al. Analysis of cell-free DNA to assess risk of tumoremia following endoscopic ultrasound fine-needle aspiration of pancreatic adenocarcinoma. Clinical Gastroenterology and Hepatology. In press.