Feb. 06, 2025
Osteoarthritis (OA) is a highly prevalent, incurable disease that carries a massive disease burden for patients and society. Affecting more than 32.5 million Americans, OA is a major cause of disability among older adults. One of the challenges encountered in treating OA is the difficulty of delivering sustained, therapeutic concentrations of anti-arthritic agents, especially biologics, to individual joints damaged by OA. Because there are few effective Food and Drug Administration-approved drugs for OA, many patients progress to the point of needing an artificial joint.
Gene therapy is an exciting avenue of research offering the potential for an effective nonsurgical approach to treating OA. More than 30 years ago, researcher Christopher H. Evans, Ph.D., and colleagues proposed that local, intra-articular gene transfer could deliver therapy to damaged joints by engineering articular cells to synthesize anti-arthritic gene products endogenously. Dr. Evans holds joint appointments in Physical Medicine and Rehabilitation, Orthopedics, and Molecular Medicine at Mayo Clinic in Rochester, Minnesota, and leads Mayo Clinic's Rehabilitation Medicine Research Center and Musculoskeletal Gene Therapy Research Laboratory.
In a review article published in the Journal of the American Academy of Orthopaedic Surgeons in 2024, Dr. Evans and colleagues summarize the progress made in the development of intra-articular gene therapy. This article highlights some key milestones in the promising trajectory of this research and describes the next steps in this novel approach to treating OA.
How the gene therapy approach works
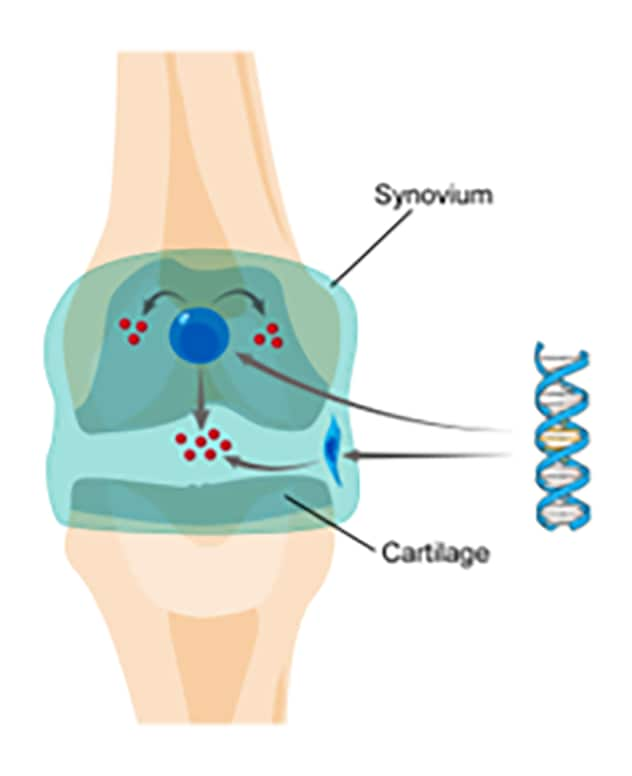 How gene therapy works
How gene therapy works
Dr. Evans and colleagues have developed a viral vector containing DNA that encodes a therapeutic gene product. When this vector is injected into the affected joint, it delivers its genetic payload to the synovial lining cells and articular chondrocytes. These cells then synthesize the therapeutic gene product locally in a sustained fashion.
"The basic concept is quite simple," explains Dr. Evans. "We have developed a viral vector containing DNA that encodes a therapeutic gene product. When this vector is injected into the affected joint, it delivers its genetic payload to the synovial lining cells and articular chondrocytes. These cells then synthesize the therapeutic gene product locally in a sustained fashion."
Although the concept may seem simple, gene therapy as a whole was in its infancy when Dr. Evans and colleagues started this project. And over the past three decades, Dr. Evans and other gene therapy researchers have encountered multiple hurdles. After overcoming many of those barriers to progress, Dr. Evans and colleagues have had much progress to report.
Key research milestones
- Identification of protein called interleukin-1 receptor antagonist (IL-1Ra) as a potential therapeutic agent in OA. IL-1Ra is a natural inhibitor of interleukin-1 (IL-1), a key driver of inflammation, pain and cartilage loss.
- Development of vectors based on adeno-associated virus (AAV) that are safe, effective, relatively stable and able to transfer genes to cells in situ.
- Successful delivery of self-complementary AAV encoding IL-1Ra cDNA (scAAV.IL-1Ra) via intra-articular injection to the joints of experimental animals in preclinical studies that confirmed safety and efficacy.
- A phase 1 trial conducted at Mayo Clinic that confirmed the safety and tolerability of using scAAV.IL-1Ra, introduced by intra-articular injection into human knee joints with moderate OA. An abstract highlighting the findings was published in Molecular Therapy in 2022, and a full manuscript has been submitted for publication.
Next steps
Dr. Evans notes that a larger, multicenter phase 1b clinical trial (ClinicalTrials.gov identifier NCT05835895) is underway to evaluate further the safety, pharmacokinetics and biomarker effect of scAAV.IL-1Ra in patients with knee OA.
After years of work to overcome challenges, Dr. Evans is hopeful that successful completion of the phase 1b trial in the second quarter of 2025 and subsequent larger trials will push the team closer to seeing scAAV.IL-1Ra licensed for use in a clinical setting for the treatment of OA. He also believes that this approach may be of therapeutic value for other diagnoses.
"Beyond OA, gene therapy could be effective for a wide range of degenerative, inflammatory and fibrotic conditions that affect the nervous system, cardiovascular system, respiratory system and other organ systems," says Dr. Evans.
For more information
Evans CH, et al. The 2024 OREF Clinical Research Award: Progress toward a gene therapy for arthritis. Journal of the American Academy of Orthopaedic Surgeons. 2024;32:1052.
Sellon JL, et al. A phase 1 clinical trial of osteoarthritis gene therapy (NCT02790723). Molecular Therapy. 2022;30:376.
Refer a patient to Mayo Clinic.