April 17, 2020
Adrenal masses are found in up to 7% of the population undergoing abdominal imaging, but adrenal tumors larger than 4 cm represent a small proportion of these tumors. Adrenocortical carcinoma (ACC) and other malignant adrenal tumors ― such as metastases, lymphoma and rarely sarcoma ― represent 13% and 18% of adrenal masses larger than 4 cm evaluated in endocrine practice, respectively, as reported in research published in Mayo Clinic Proceedings: Innovations, Quality & Outcomes in 2018.
Tumor size (larger than 4 cm, and particularly larger than 10 cm) as well as unenhanced Hounsfield units (HU) on computerized tomography (larger than 20 or increased heterogeneity) are strong predictors of malignancy. History of extra-adrenal malignancy is associated with a diagnosis of adrenal metastasis, while a hormonally active adrenal mass (especially combined cortisol and androgen excess) is strongly suggestive of ACC.
Adrenal biopsy is not recommended in any adrenal tumor suspicious for ACC, mostly due to poor accuracy and concern for needle track seeding, as reported in research published in Clinical Endocrinology and the European Journal of Endocrinology in 2016. Furthermore, adrenal biopsy is an invasive and typically expensive procedure and is contraindicated in any adrenal mass suspicious for pheochromocytoma.
Steroid profiling, both in serum and urine samples, is currently clinically available and can provide valuable data to guide appropriate next steps in management, as reported in research published in Clinical Chemistry in 2017 and Endocrine Practice in 2019. As demonstrated in the following cases, steroid profiling is especially helpful in two clinical scenarios:
- Distinguishing a lipid-poor adenoma from a smaller ACC to decide on laparoscopic vs. open adrenalectomy
- Distinguishing ACC from other malignant adrenal masses
Case 1
Carcinoma corticosuprarrenal (caso 1)
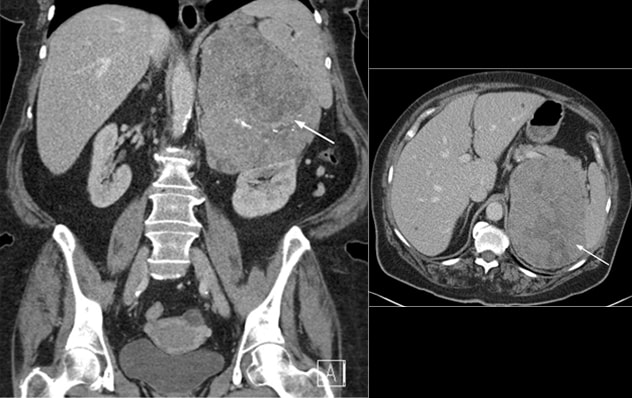
Carcinoma corticosuprarrenal (caso 1)
Carcinoma corticosuprarrenal (caso 1) en las vistas coronal (izquierda) y transversal (derecha) de la tomografía computarizada del abdomen
A 71-year-old woman was evaluated by her primary care physician for symptoms of worsening shortness of breath. A computerized tomography (CT) scan of the chest did not show any lung pathology but identified an incidental left adrenal mass. Subsequent CT of the abdomen revealed a 15-by-12-cm well-circumscribed heterogeneous left adrenal mass with scattered dystrophic calcifications and marked local mass effect. No other lesions were noted in the abdomen or the chest.
The patient had a known diagnosis of sleep apnea, hypertension, type 2 diabetes mellitus, obesity and dyslipidemia. She reported no personal or family history of any genetic syndrome or malignancy. During evaluation in the adrenal clinic, she was asymptomatic and denied any changes to appetite or weight.
Biochemical workup of 2 patients
Serum and urine profiling (case 1)
On physical exam, no features of overt Cushing syndrome, acne, hirsutism, androgenic alopecia or edema were noted. Biochemical workup was consistent with ACTH-independent cortisol excess and androgen excess, without evidence for primary aldosteronism or catecholamine excess. Preoperative serum and urine steroid profiling were consistent with adrenocortical carcinoma, with elevated androgen and glucocorticoid precursors and metabolites. The patient underwent an open left adrenalectomy, and surgical pathology confirmed ACC (Ki-67 index of 70%).
Case 2
A 65-year-old woman was evaluated for unexplained persistent low-grade fever associated with mild neutrophilia. Her medical history included hypertension, dyslipidemia and prediabetes. In addition, she had a history of stage I uterine cancer for which she underwent a hysterectomy two years prior to the current evaluation.
Leiomiosarcoma poco diferenciado (caso 2)
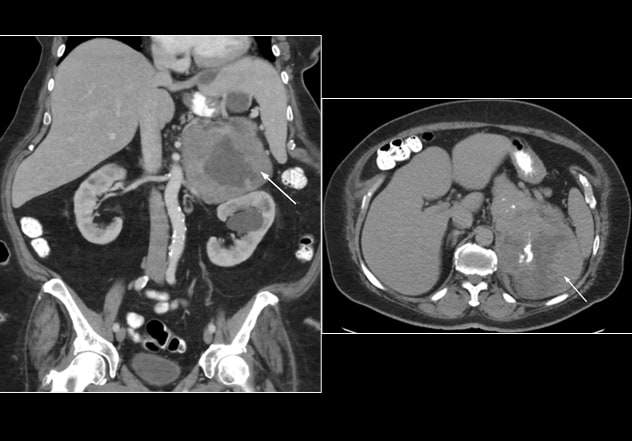
Leiomiosarcoma poco diferenciado (caso 2)
Leiomiosarcoma mal diferenciado (caso 2) visto en las vistas coronal (izquierda) y transversal (derecha) de la tomografía computarizada del abdomen
The patient had no personal history of genetic syndrome or family history of malignancy. Following an episode of fever with abdominal pain, she underwent a CT of the abdomen that incidentally identified a 20-by-17-by-10.5-cm large mixed solid and cystic mass in the left adrenal gland, with scattered calcifications abutting surrounding structures.
On evaluation in the adrenal clinic, mild hirsutism was noted without evidence of acne, androgenic alopecia, features of Cushing syndrome or edema. Biochemical workup revealed abnormal dexamethasone suppression test, but otherwise no hormonal excess. Preoperative serum and urine steroid profiling were not suggestive of ACC. Open left adrenalectomy was performed, and pathology demonstrated a poorly differentiated leiomyosarcoma.
Discussion
These two cases of malignant adrenal tumors are both highly suspicious for ACC based on imaging and standard of care hormonal work-up. Serum and urine steroid profiles of the patient in case one were highly suggestive of ACC, but those of the patient in case two favored other diagnoses, such as metastases or lymphoma. This additional information allowed for individualized approaches to confirm the diagnoses in both patients.
Preoperative diagnosis of ACC will guide a recommendation for open adrenalectomy without delay in case one, whereas excluding ACC as in case two may lead to reconsideration of adrenalectomy in favor of adrenal biopsy or other diagnostic tests.
Serum and urine steroid profiling should be considered in any patient with indeterminate adrenal mass (tumor size larger than 4 cm, HU > 10 and especially HU > 20) prior to surgery to help guide subsequent diagnostic and therapeutic measures.
For more information
Bahn C. A breakthrough in distinguishing benign adrenal tumors from cancerous ones. Mayo Clinic.
Iniguez-Ariza NM, et al. Clinical, biochemical, and radiological characteristics of a single-center retrospective cohort of 705 large adrenal tumors. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 2018;2:30.
Delivanis DA, et al. Procedural and clinical outcomes of percutaneous adrenal biopsy in a high-risk population for adrenal malignancy. Clinical Endocrinology. 2016;85:710.
Fassnacht M, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. European Journal of Endocrinology. 2016;175:G1.
Hines JM, et al. High-resolution, accurate-mass (HRAM) mass spectrometry urine steroid profiling in the diagnosis of adrenal disorders. Clinical Chemistry. 2017;63:1824.
Athimulam S, et al. Clinical applicability of serum steroid precursors in the diagnosis of adrenal tumors and Cushing syndrome. Endocrine Practice. 2019;25:15.