Nov. 19, 2020
Tracheobronchial stents have been available since the 1980s. The initial stents were made from silicone molded into straight hollow tubes or fashioned in a Y configuration to fit the main carina, as described by Jean-François Dumon, M.D., in Chest in 1990.
In the past 30 years many other airway stent designs have been developed, including bare metal stents, hybrid stents that have both metal and nonmetal components, and balloon expandable stents. Despite these advances, silicone stents of the same design used for decades remain a preferred stent in some cases due to ease of removal, no risk of metallic fracture and some ability for stent customization. However, traditional silicone stents do not conform as well to the airway as self-expanding metallic stents, which can limit their utility.
Regardless of the stent type, stent-related complications such as obstructive granulation tissue formation, migration, erosion, stent fracture, infection and mucus plugging remain common. Because of these complications, stents are often one of the last options used for both malignant and nonmalignant stenosis of the trachea and bronchi. However, in the right patient, a stent plays a critical role in maintaining airway patency.
Tracheobronchial stenosis can originate from many etiologies; malignancy, post radiation, vasculitis, mediastinal fibrosis, and post lung transplant or other lung surgery are the most commonly encountered etiologies. Because of this varied origination, the configuration and fit of an ideal stent are likely unique to an individual patient. Until very recently, achieving proper stent sizing for an individual patient was met by having a wide assortment of standard stent types and sizes available and matching that to the patient's anatomy.
FDA-approved, patient-specific airway stents
左主干支气管远端
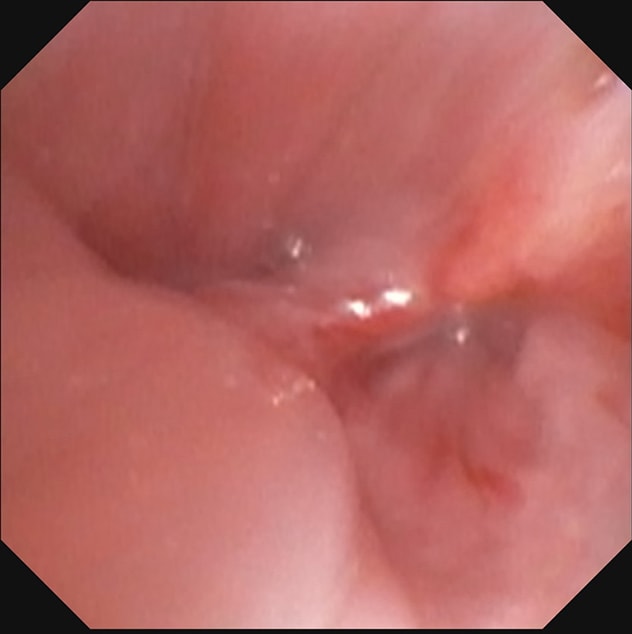
左主干支气管远端
左上肺叶袖状切除术部位的左主干支气管远端。
严重狭窄区域和支架
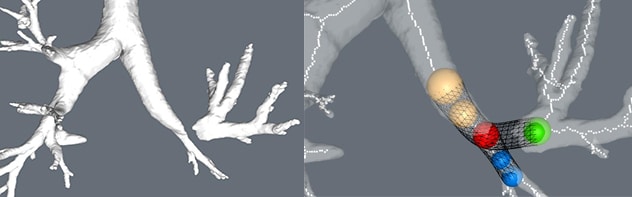
严重狭窄区域和支架
左上肺叶袖状切除术部位的严重狭窄区域(左图)。支架建模(右图)。
患者特定硅胶支架
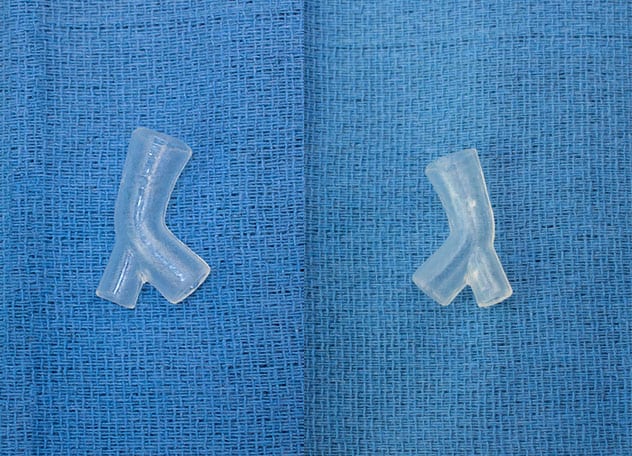
患者特定硅胶支架
患者特定硅胶支架的正面和背面视图。
扩张后和支架插入的近端和远端视图
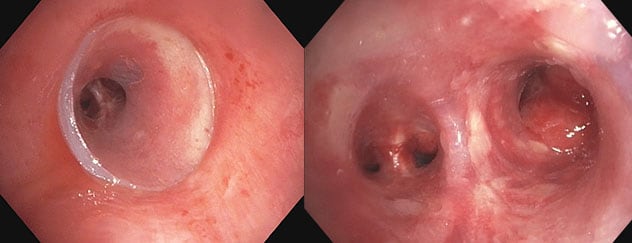
扩张后和支架插入的近端和远端视图
扩张后和支架插入的近端和远端视图。虚拟建模技术允许未来在需要时进行轻微的改动。
The past several years have seen an explosion of new medical technologies. Advances in thin slice CT scanning, 3D virtual airway modeling and 3D printing have converged to now allow doctors to design custom silicone airway stents. Mayo Clinic is one of the first centers to be able to offer patients with complex central airway stenosis the first patient-specific, customized airway stents approved by the Food and Drug Administration (FDA). These stents are available as part of a controlled beta launch.
According to Ryan M. Kern, M.D., an interventional pulmonologist at Mayo Clinic in Rochester, Minnesota: "With this exciting new technology, we can take a patient's CT scan, build a 3D virtual model and rebuild areas of airway narrowing. This virtual model is then made into a 3D-printed mold and medical-grade silicone is injected to create a patient-specific silicone airway stent. As the airway is treated, the model can be further altered by making incremental changes if needed. Implantation of the stent is performed with rigid bronchoscopy. The technology allows physicians to employ tried-and-true materials and techniques with sophisticated patient-specific design."
For more information
Dumon JF. A dedicated tracheobronchial stent. Chest. 1990;97:328.
VisionAir 3D Stent Architect. VisionAir Solutions.