April 10, 2014
The complex biology of myostatin inhibition
饮食诱发的肥胖症衰老小鼠的肌肉生长抑制素治疗方法的结果
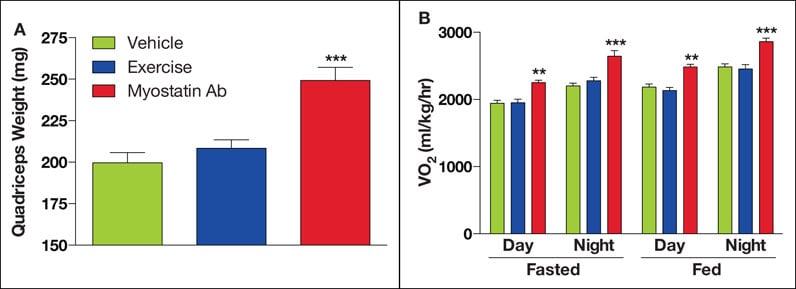
饮食诱发的肥胖症衰老小鼠的肌肉生长抑制素治疗方法的结果
在饮食诱发的肥胖症衰老小鼠中,与对照组相比,进行短期(每周 1 次,持续 6 周)肌肉生长抑制素抗体 (Ab) 治疗会引起股四头肌重量显著增加 (A)。而且,根据测量耗氧气量 (VO2) 显示,由于抑制肌肉生长的抑制素使得瘦体重增加,从而导致在进食和禁食状态下能量消耗都更大 (B)。适度的耐受运动没有显著影响这些结果。符号 ** 和 *** 分别表示 p <0.01 和 0.001。
Most adult humans achieve peak muscle mass sometime during their early 40s. After that point, a gradual deterioration begins. The progressive loss of skeletal muscle mass that accompanies aging (sarcopenia) and disease (cachexia) can impair muscle performance, physical function and whole-body metabolism. The declines in physical function and mobility associated with sarcopenia and cachexia can lead to falls, loss of independence, institutionalization and even death. Given the severity of these outcomes, current research seeks to gain a better understanding of the biology of sarcopenia and cachexia and initiate development of therapeutic interventions to prevent, slow or reverse their progression.
The biological mechanisms underlying sarcopenia and cachexia are not well-understood. But researchers have established that multiple factors play a role, including age-associated hormone changes, sex steroids, physical inactivity, inflammation, and comorbid conditions such as heart failure, cancer and diabetes.
"Without question, exercise is the most powerful intervention to address muscle loss, whether it occurs in the context of advancing age or debilitating chronic or acute diseases," explains Nathan K. LeBrasseur, Ph.D., of the Department of Physical Medicine and Rehabilitation at Mayo Clinic in Rochester, Minn. "However, researchers are also searching for pharmacological therapies to help improve skeletal muscle mass among people who are bedbound or unable to exercise for other reasons."
The discovery that the growth and differentiation factor-8 (GDF-8), also known as myostatin, functions as a potent negative regulator of muscle growth has led to the exploration of whether it can serve as a mediator of sarcopenia or cachexia and as a therapeutic target. Researchers have observed that deletion and loss of function mutations in myostatin cause an increase in the number of skeletal muscle fibers (skeletal muscle hyperplasia) and an increase in the size of skeletal muscle fibers (hypertrophy). These observations have led to the hypothesis that myostatin inhibition could serve as a means to attenuate or reverse skeletal muscle mass loss in patients affected by sarcopenia, cachexia and genetic disorders such as muscular dystrophy.
Measurement of myostatin abundance is difficult, and the fact that this measurement may or may not reflect its activity further complicates the picture. "Recent research has yielded disparate findings about the relationship between age and the abundance or activity of myostatin, and about whether myostatin is a primary driver in sarcopenia," says Dr. LeBrasseur. "More-advanced techniques to quantify the mature (biologically active) and inactive forms of this factor will need to be developed before we can draw clear conclusions about myostatin's true role in sarcopenia."
Dr. LeBrasseur and colleagues are currently developing the methodology to precisely measure myostatin, and they have started analyzing the data obtained from testing in 240 subjects.
Exploring myostatin's therapeutic potential
It's true that myostatin's expression and activity patterns during aging are incompletely understood. However, recent research has highlighted several characteristics that make it a promising therapeutic target for sarcopenia:
- Myostatin inhibition, even partial reductions, increases muscle mass in adult and older mammals.
- Myostatin's effects are highly specific to muscle mass.
- Disrupting myostatin signaling may also positively affect multiple other age-associated changes, including increased bone mineral density, improved cardiac ejection fraction, and resistance to diet-induced obesity, dyslipidemia, atherogenesis, hepatic steatosis and inflammation.
- Myostatin is a highly druggable protein because it is secreted and accessible in the circulation.
Researchers are investigating the use of antibodies, propeptides, interacting proteins and soluble decoy receptors to inhibit myostatin activity. "Several studies offer evidence that while multiple strategies do inhibit myostatin, their safety, specificity and effectiveness differ," explains Dr. LeBrasseur. Studies in mice and humans using a soluble decoy receptor of myostatin as an anabolic intervention, for example, have demonstrated some negative side effects.
Dr. LeBrasseur notes that while these and other studies have provided promising results, future research needs to establish the optimal way to inhibit myostatin and safely increase muscle mass.
For more information
White TA, et al. Myostatin and sarcopenia: Opportunities and challenges — A mini-review. Gerontology. In press.
Points to remember
- Myostatin regulates muscle development and postnatal growth.
- Inhibition of myostatin in adult and older animals significantly increases muscle mass and improves muscle performance and metabolism.
- These effects, along with the relative exclusivity of myostatin to muscle and the effects of its targeted inhibition on muscle, make it a promising drug target for sarcopenia.
- More research is needed to determine the best means by which to disrupt the activity of myostatin and related factors to safely increase muscle mass.