Jan. 17, 2020
A 24-year-old woman with type 1 diabetes mellitus presented at Mayo Clinic to establish care. She was diagnosed with diabetes at age 7 years after developing diabetic ketoacidosis. The patient was using a hybrid closed-loop insulin delivery system including an insulin pump and a continuous glucose monitor. The pump was in automatic mode, using a target of 120 mg/dL to adjust basal insulin delivery. HbA1c was 7.3% with minimal hypoglycemia, and while there were no known microvascular or macrovascular complications, the last screening was completed 12 months prior to this visit.
An intrauterine contraceptive device was in place, but the patient expressed a desire to have it removed and become pregnant in the near future. She had no prior pregnancies.
Discussion
高血糖—高胰岛素血症 (Pedersen) 假设
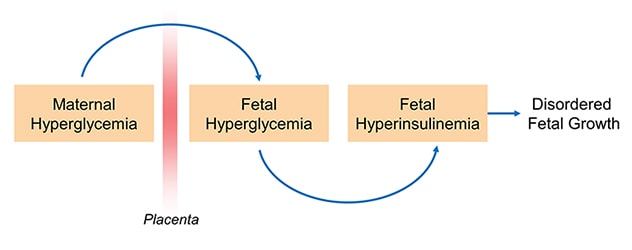
While the vast majority of women with diabetes have positive pregnancy outcomes, extra care and attention is required to achieve that outcome. Maternal glucose can diffuse to the fetal circulation and result in fetal hyperglycemia, which in turn promotes fetal hyperinsulinemia and contributes to abnormal growth and development.
Diabetes during pregnancy may be classified as pre-gestational (including type 1 and type 2) or gestational diabetes mellitus. This patient fell into the former category, as diabetes was established prior to conception. Women with pre-gestational diabetes are at increased risk of gestational hypertension and cesarean delivery. Their infants are at increased risk of:
- Congenital anomalies
- Birth injuries (associated with shoulder dystocia)
- Being large for gestational age
- Developing neonatal hypoglycemia
Structured programs providing coordinated, evidence-based care to women with diabetes before and during pregnancy, such as the program outcomes reported in The Journal of Clinical Endocrinology and Metabolism in 2016, have demonstrated improved clinical outcomes. In particular, the value of targeted pre-pregnancy care is well established. Pre-pregnancy care, including glycemic control right through conception and the critical early stages of pregnancy when organogenesis is taking place, ensures optimal maternal health. Typically, patients are reviewed every one to three months in the outpatient setting and contraception is advised until treatment goals are achieved.
Components of pre-pregnancy care
怀孕前护理的关键组成部分
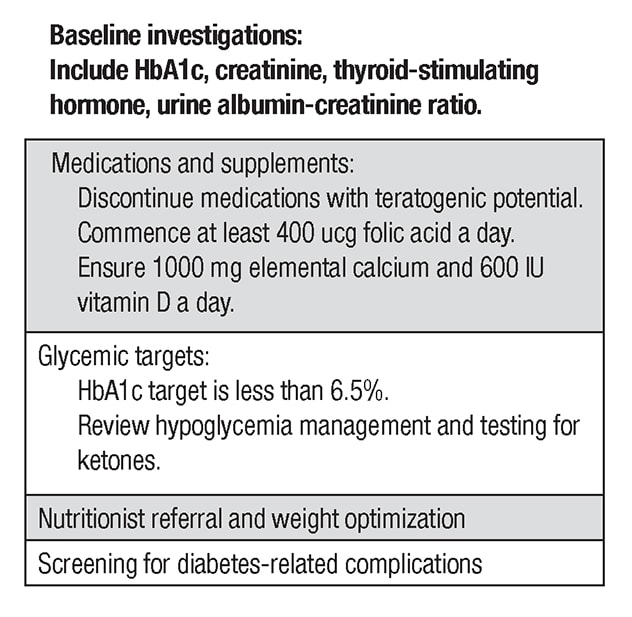
Among the key components of pre-pregnancy care, a full medication review should occur and medications with teratogenic potential such as angiotensin-converting enzyme inhibitors or statins should be discontinued. Women should take at least 0.4 mcg of folic acid per day for a minimum of three months prior to conception. Baseline labs including thyroid function tests should be performed. Retinal screening and assessment for nephropathy and neuropathy should occur within a year of pregnancy. In certain clinical situations, screening for macrovascular complications may be required. Pregnancy should be deferred until complications are treated.
With the exception of metformin and insulin, all glucose-lowering agents should be discontinued. Previously, NPH insulin and regular insulin were typically used in this setting. Currently, rapid-acting insulins including insulin aspart and insulin lispro are considered safe and are commonly used before and during pregnancy. The long-acting insulin analogues insulin detemir and insulin glargine also appear safe and are frequently used in clinical practice.
There are no significant data to support use of other insulins during pregnancy. If possible, an HbA1c of less than 6.5% should be targeted pre-pregnancy, as rates of congenital anomalies drop to close to those of the background population in this setting. In order to achieve this goal, patients should target a fasting glucose of less than 95 mg/dL and a two-hour postprandial glucose of less than 120 mg/dL.
A randomized controlled trial (CONCEPTT) recently demonstrated that the use of continuous glucose monitoring during pregnancy in patients with type 1 diabetes is associated with improved neonatal outcomes, and this technology may be initiated in the pre-pregnancy setting if clinically appropriate. Study results were published in The Lancet in 2017.
At Mayo Clinic, the patient was followed in clinic regularly. She worked with members of the multidisciplinary team, including a dietitian and a nurse educator. The insulin pump was switched to manual mode to tighten glycemic control, and screening for complications was completed.
The patient's thyroid-stimulating hormone level was 4.0 mIU/L. Levothyroxine was introduced to target a thyroid-stimulating hormone level of 0.1 to 2.5 mIU/L, which is considered optimal for the first trimester of pregnancy. At six months, the patient's HbA1c had improved to 6.2% and her intrauterine device was removed.
Three months later the woman became pregnant. She was followed closely during pregnancy by providers in Obstetrics and Endocrinology. She developed preeclampsia at 35 weeks of gestation. After induction of labor at 37 weeks, she delivered a healthy baby girl weighing 3.3 kg. The vaginal delivery was normal and there were no neonatal complications.
For more information
Egan AM, et al. A prepregnancy care program for women with diabetes: Effective and cost saving. The Journal of Clinical Endocrinology and Metabolism 2016;101:1807.
Feig DS, et al. Continuous glucose monitoring in pregnancy women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. The Lancet. 2017;390:2347.