Feb. 12, 2022
Colonoscopy is the ideal screening examination for colon polyps and colon cancer, as it allows physicians to detect and remove precancerous and sometimes cancerous lesions from the colon. One of the most common complications of colon polyp removal is bleeding from the site of polyp removal. Closing a tissue defect is sometimes accomplished using endoscopic clips (either through the scope or over the scope) or via endoscopic suturing. These approaches have proved effective in significantly reducing the risk of bleeding in many instances. However, when closing large and challenging defects that have the highest risk of bleeding, both clips and endoscopic suturing systems have limitations that can make them less ideal for this task.
经内镜缝合器
经内镜缝合器
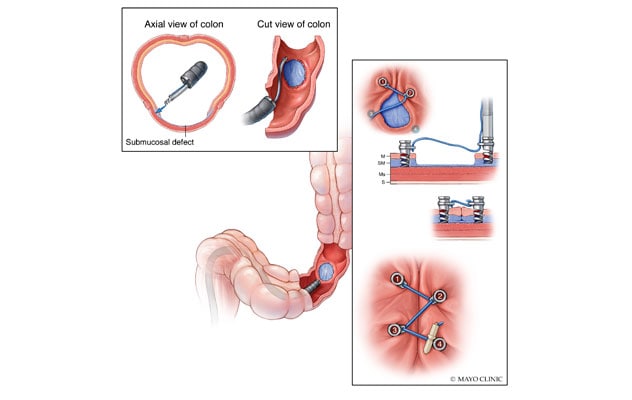
经内镜缝合器有四个 5 毫米的钢制外科螺旋钉,通过每个螺旋钉中心点附近的孔眼拴在一根缝合线上。内镜医生通过任何市售的胃镜或结肠镜,依次放置螺旋针,闭合组织缺损,防止延迟性出血。
In December 2020, the U.S. Food and Drug Administration (FDA) approved the use of a novel tack and suture device, designed by the Developmental Endoscopy Unit at Mayo Clinic in Rochester, Minnesota, to address these difficult tissue closures. This novel tack and suture system facilitates closure of challenging defects via endoscopic placement of tacks that are drilled into adjacent healthy tissue. The device has four 5-mm surgical steel helix tacks tethered on a single 3-0 polypropylene suture that runs through an eyelet near each tack's midpoint. Endoscopists deploy the tacks sequentially through a channel (at least 2.8 mm in diameter) of any commercially available gastroscope or colonoscope.
A team of researchers in the Developmental Endoscopy Unit at Mayo Clinic in Rochester, Minnesota, studied the device before its FDA approval, and they subsequently began clinical use at Mayo Clinic in January of 2021. In an article published in Gastrointestinal Endoscopy in 2022, Mayo Clinic researchers and co-investigators from seven other sites within the U.S. detail their initial experiences using the tack and suture device. The study group included the team from Mayo Clinic and investigators from Johns Hopkins Medicine, Harvard Medical School and several other sites across the United States.
The research team collected and analyzed data from device use in 93 patients (48.4% female) with a mean age of 63.6 ± 13.1 years. Primary outcomes for this retrospective multicenter study included feasibility and safety of early use of the device. Secondary outcomes included assessment of need for additional closure devices, prolonged procedure time and technical feasibility of performing the procedure with alternative devices.
Results
According to co-authors Andrew C. Storm, M.D., and Tala Mahmoud, M.D., the study yielded a number of interesting findings related to the efficiency and safety of the tack and suture device. Dr. Storm is a gastroenterologist at Mayo Clinic in Minnesota and part of the research team that studied the device before its FDA approval. Dr. Mahmoud is a Mayo Clinic gastroenterology research fellow and first author on the published study.
- Technical success was achieved in 83 patients (89.2%), and supplemental closure was required in 23 patients (24.7%) with a mean defect size of 41.6 ± 19.4 mm.
- In 24.7% of the patients, closure with something other than the device under study (using previously available endoscopic clips, etc.) would not have been possible due to defect location, size or shape.
- The use of the tack and suture device prolonged the procedure (mean duration of 12.8 ± 3.9 minutes per system) in 8.6% of the cases. But endoscopists reporting this data considered this amount of time acceptable.
- Adverse events (AEs) occurred in 2.2% of the cases during a 34-day follow-up period (interquartile range: 13 to 93.5 days) and were mild and moderate in severity. No serious AEs or procedure-related deaths occurred.
"Overall, this study demonstrated that this through-the-scope suturing device was able to successfully close a wide range of challenging tissue defects," explains Dr. Storm. "This tool addresses an unmet need in gastrointestinal tissue closure, allowing endoscopists to close tissue after very large and challenging polyp removal procedures. Future studies will help clinicians determine other uses for this novel tack and suture device."
For more information
Mahmoud T, et al. Initial multicenter experience using a novel endoscopic tack and suture system for challenging GI defect closure and stent fixation (with video). Gastrointestinal Endoscopy. 2022;95:373.
Refer a patient to Mayo Clinic.