Aug. 22, 2018
Sarcoidosis is a multisystem inflammatory disorder of unknown origin that is characterized by noncaseating granulomas in involved tissues. It most commonly affects young and middle-aged adults. Sarcoidosis frequently presents with bilateral hilar lymphadenopathy, pulmonary infiltrates, uveitis or skin lesions, but any organ, including the heart, may be involved. Only a small minority of patients with sarcoidosis are diagnosed with cardiac involvement. According to autopsies of a series of patients with systemic sarcoidosis, however, the rate of cardiac sarcoidosis (CS) is approximately 25 percent, indicating that CS is underdiagnosed in this population.
Diagnosing CS
心脏结节病心脏活检的显微照片(20 倍物镜)
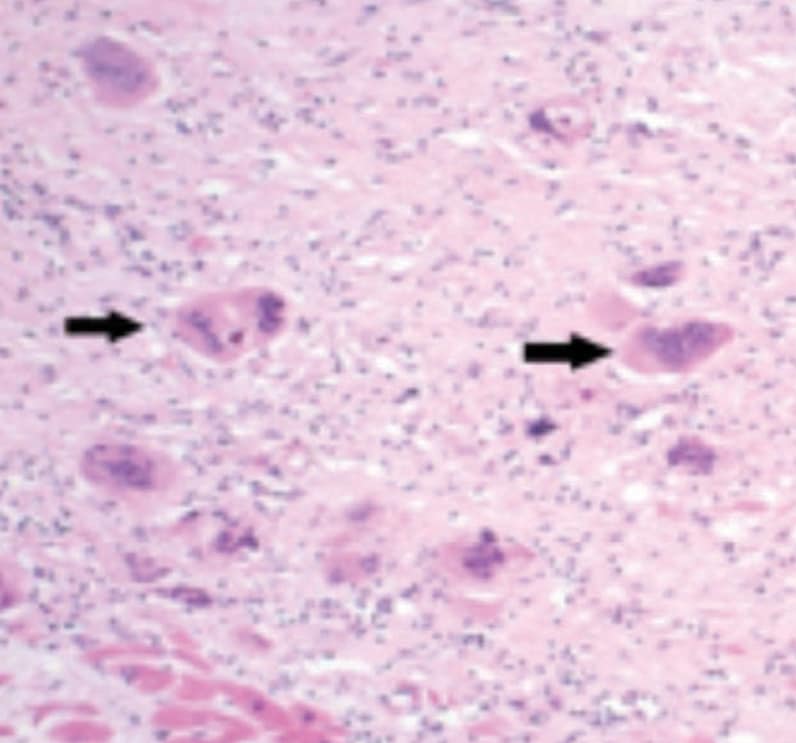
心脏结节病心脏活检的显微照片(20 倍物镜)
心脏结节病的特征性组织病理学检查结果,包括在致密的替代型纤维化背景中伴多个多核巨细胞(箭头处)浸润的非坏死性肉芽肿性炎症反应。
Diagnosing CS remains challenging. Definite CS can only be established by the presence of noncaseating granulomas on endomyocardial biopsy (EMB).
Due to the patchy nature of granulomatous deposition in the myocardium, the diagnostic yield of EMB has been reported to be only about 20 percent. Using electrogram guidance, as is commonly performed during EMB for suspected CS at Mayo Clinic, increases the diagnostic yield up to 50 percent. Suraj Kapa, M.D., an electrophysiologist at Mayo Clinic's campus in Rochester, Minnesota, and his colleagues are continuing to work on optimizing where, when and how biopsies should be performed to confirm cases of CS.
Cardiac involvement may precede, occur concurrently with, or follow lung or other organ involvement. Clinicians should thus consider the possibility of CS in patients with known extracardiac sarcoidosis who develop cardiac symptoms, echocardiographic changes or abnormal findings on cardiac imaging. Clinicians should also consider the diagnosis of CS in otherwise healthy young or middle-aged persons who present with cardiac symptoms, arrhythmias or heart failure without a preceding diagnosis of extracardiac sarcoidosis. A high index of suspicion is thus required to consider the diagnosis of CS, and expert evaluation is essential to confirm the diagnosis and provide appropriate recommendations regarding therapy.
心脏结节病患者的经胸超声心动图成像
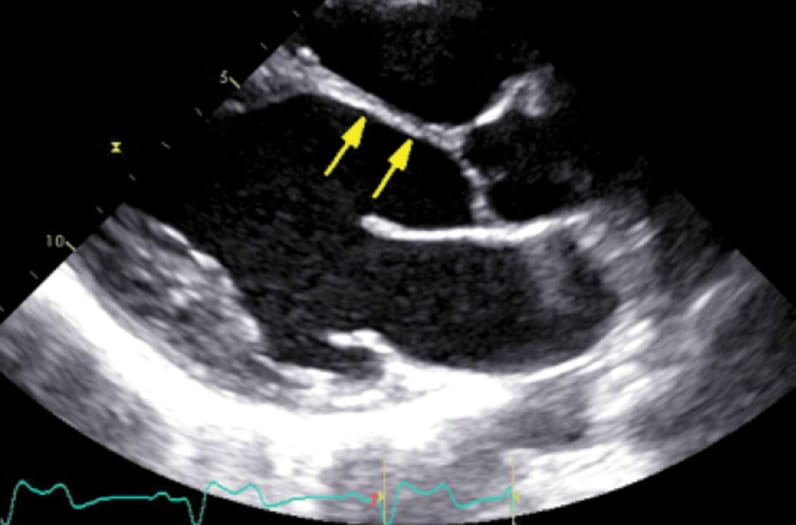
心脏结节病患者的经胸超声心动图成像
胸骨旁长轴图显示前间隔基底段和中室段变薄,这是晚期心脏结节病的一个特征表现。
心脏结节病患者的心脏磁共振成像平扫图
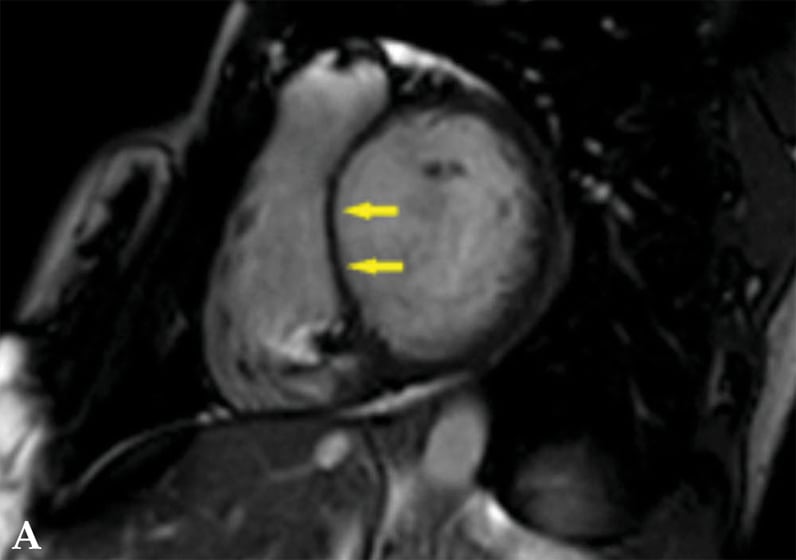
心脏结节病患者的心脏磁共振成像平扫图
平扫图显示左心室扩张伴室间隔明显变薄(箭头处)。
心脏结节病患者注入对比剂后的心脏磁共振成像
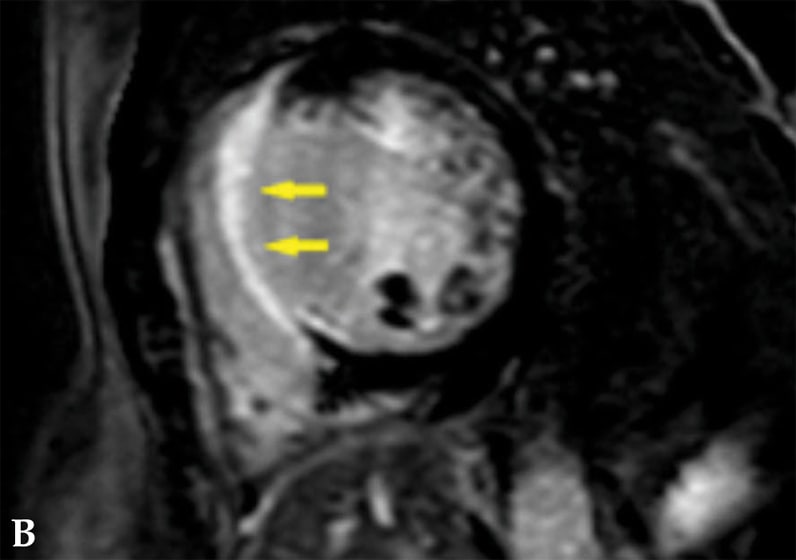
心脏结节病患者注入对比剂后的心脏磁共振成像
造影后图像显示累及整个室间隔(箭头处)的局灶透壁晚期钆增强。
氟脱氧葡萄糖活性增加的多个病灶部位
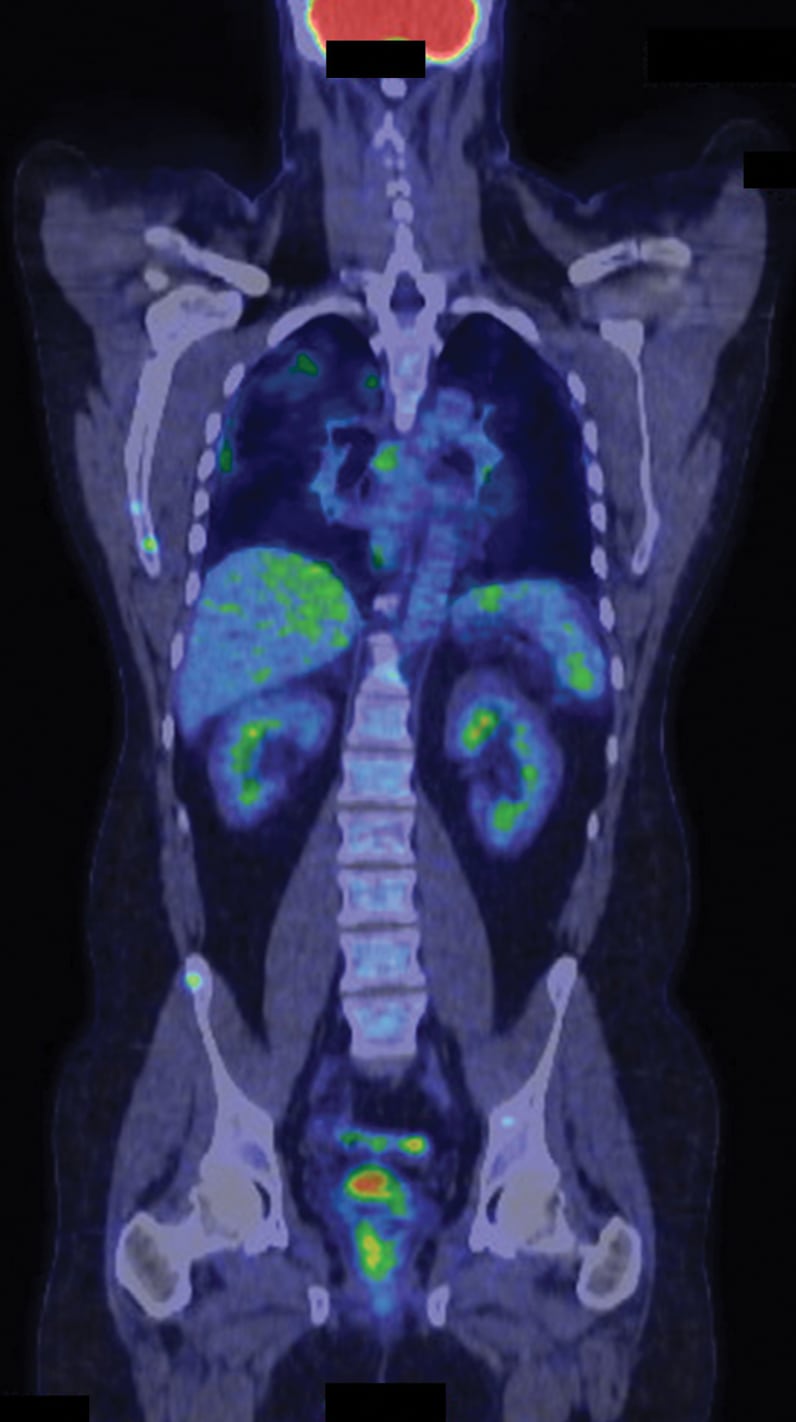
氟脱氧葡萄糖活性增加的多个病灶部位
一名心脏和心外结节病患者正在接受氟脱氧葡萄糖 (FDG) 正电子发射断层成像/计算机断层成像的影像学检查(最大密度投影)。氟脱氧葡萄糖活性增加的多个病灶部位在心脏、肺部、胸膜、肝脏、脾脏、骨骼和多个淋巴结中较为明显。
Echocardiography is often the first imaging screening test for CS and is extremely useful for assessing left and right ventricular systolic and diastolic function and estimating pulmonary artery pressures. Echocardiography, however, has low sensitivity for detecting early CS. Newer echo technologies, including speckle tracking imaging, show promise in the early diagnosis of CS and may predict clinical outcomes.
Cardiac magnetic resonance (CMR) has also emerged as a useful tool in the diagnosis of CS. Although CMR images may show thinned walls, aneurysms and segmental wall motion abnormalities in a noncoronary distribution, the principal method for detecting CS by CMR relies on identifying areas of late gadolinium enhancement, usually in a subepicardial or transmural distribution.
Cardiac FDG-PET has increasingly been utilized for the diagnosis and management of CS due to its high spatial resolution. Cardiac PET imaging for CS involves two different scans:
- One scan to assess resting myocardial perfusion and areas of fibrosis or scar using 82Rubidium or 13N-Ammonia
- Another scan to detect inflammation using fluorodeoxyglucose (FDG)
In early stages of the disease, focal areas of increased FDG uptake are present and resting perfusion defects may be seen. In advanced CS, resting perfusion defects may be seen in the absence of FDG uptake, indicating the presence of scar without inflammation. Resting perfusion defects and areas of inflammation may either coincide with or occur in different locations of the myocardium.
Whole-body FDG imaging, typically from the orbits to the mid-thigh level, is increasingly being used to evaluate for extracardiac sarcoidosis during the same PET session at which dedicated cardiac perfusion and FDG imaging occurs. Clinicians may find this useful for identifying extracardiac metabolically active areas that may be amenable to biopsy and to help discern whether or not immunosuppressive therapy is indicated.
Management
There currently are no FDA-approved therapies for sarcoidosis in general or CS in particular. "Although corticosteroids are the mainstay treatment for patients with CS, there is a paucity of data to support the effectiveness of this therapy," according to Lori A. Blauwet, M.D., director of the Cardiac Sarcoidosis Clinic at Mayo Clinic in Rochester, Minnesota. "Optimal doses and duration of corticosteroid therapy have not been systematically studied."
Although there are no clear guidelines regarding when to initiate corticosteroid therapy, most experts agree immunosuppression should be considered in symptomatic CS patients with evidence of active myocardial inflammation and any of the following:
- Reduced left ventricular ejection fraction (LVEF)
- High grade atrioventricular (AV) block
- Frequent premature ventricular contractions or frequent nonsustained ventricular tachycardia (VT)
- Sustained VT or ventricular fibrillation
It remains uncertain whether or not asymptomatic patients with CS benefit from treatment.
Dr. Blauwet is engaged in setting up clinical teams and trials to better track and assess outcomes in patients with CS who are receiving a variety of treatment options. Steroid-sparing agents are often used for refractory cases or when patients experience adverse effects from steroid therapy. Antimetabolites such as methotrexate, azathioprine, leflunomide, mycophenolate mofetil and cyclophosphamide have been used as second line agents. If the disease progresses despite use of glucocorticoids, a second line agent, or both, TNF-inhibition with either infliximab or adalimumab should be considered. Physicians at Mayo Clinic have used rituximab, a monoclonal antibody directed against the CD20 antigen on the surface of B lymphocytes, to treat patients with refractory CS, with some success.
Some centers, including Mayo Clinic, have routinely introduced a steroid-sparing agent when corticosteroid therapy is initiated rather than waiting to determine responsiveness to steroid therapy before adding a second agent, allowing a more rapid steroid taper so as to minimize the potential for steroid-induced weight gain, diabetes, osteoporosis and other complications. The steroid-sparing agent is typically continued for at least six to 12 months after prednisone has been discontinued.
"Granulomatous lesions and active inflammation are substrates for ventricular arrhythmias in patients with CS. Amiodarone may be useful, but long-term use is typically avoided due to potential lung toxicity," says Dr. Kapa.
Catheter radiofrequency ablation may be useful in some CS patients with VT, but results have been mixed. Current guidelines provided by the Heart Rhythm Society state that catheter ablation can be useful in patients with CS and ventricular arrhythmias that are refractory to immunosuppressive and anti-arrhythmic therapy. VT recurrences are common, however. Repeat ablation with concomitant anti-arrhythmic therapy in these patients may be useful.
As many patients with CS present with high-grade AV block, pacemaker implantation is frequently indicated per standard device guidelines. Device implantation can also be useful for patients with CS with an indication for pacing even if the AV block is transient. ICD implantation can be considered in patients with CS who have an indication for permanent pacing even in the absence of ventricular arrhythmias. ICD implantation is indicated in patients who have a history of spontaneous sustained ventricular arrhythmias or who have LVEF ≤ 35 percent despite optimal medical therapy and a period of immunosuppression, if inflammation is present. ICD implantation may be useful for patients with CS with unexplained syncope or near syncope and inducible sustained ventricular arrhythmias.
Prognosis
The natural history and prognosis of patients with CS remains uncertain. Symptomatic patients seem to have a poorer outcome than asymptomatic patients. The extent of LV dysfunction tends to be the most important predictor of survival.
Several studies have examined the prognosis of clinically silent CS with varying results. Many patients with clinically silent CS have a benign clinical course, but some of these patients present with sudden cardiac death. Clinicians are advised to closely monitor patients with CS for disease progression and development of symptoms so as to optimize management in order to prevent potentially devastating complications.
Mayo Clinic in Rochester, Minnesota, launched the Cardiac Sarcoidosis Clinic in 2017 in order to optimize the diagnosis and treatment of patients with this disease. The clinic is staffed by specialists from Cardiovascular Medicine with expertise in myocardial diseases and heart failure. These specialists collaborate with specialists from other disciplines, including electrophysiology, cardiac imaging, pathology, rheumatology, pulmonary, ophthalmology and neurology to provide multidisciplinary assessment and coordination of care of patients with cardiac sarcoidosis. The clinic is one of only a handful of clinics in the world specifically dedicated to the care and treatment of patients with CS.
Innovation in cardiac sarcoidosis care at Mayo Clinic
The team at the Mayo Clinic sarcoidosis clinic is involved in innovating approaches to diagnosis and treatment of patients with suspected or clinically definite cardiac sarcoidosis. These efforts have included:
- Development of a unique agent for PET scanning intended to improve diagnostic accuracy
- Development of new tools and techniques for biventricular biopsy
- Engagement with colleagues in genetics and pathology to better understand the potential pathophysiologic causes of CS and to help identify potential new therapeutic targets
Epidemiologic efforts are also underway to help clinicians understand treatment effects, elucidate patients who most benefit with specific interventions such as ablations or defibrillators, and help guide patients on the prognosis related to their specific disease.
For more information about the Cardiac Sarcoidosis Clinic at Mayo Clinic in Rochester, Minnesota, call 507-538-6388.