Dec. 18, 2020
Mayo Clinic researchers studying germline genetic alterations among patients with solid tumor cancer found that universal multigene panel testing was associated with increased detection of heritable variants over the predicted yield of targeted testing based on guidelines.
In the two-year Interrogating Cancer Etiology Using Proactive Genetic Testing (INTERCEPT) cohort study of 2,984 unselected patients with cancer, universal germline genetic testing found that 13.3% harbored a pathogenic germline variant (PGV) — and 48% of those PGVs would not have been detected using standard guidelines. Nearly 30% of patients with a high-penetrance PGV received modifications to their treatment based on the finding.
"Hereditary factors play a key role in the risk of developing several cancers. We wanted to study the impact of broad-based testing for inherited germline variants in patients with cancer, compared with more traditional approaches of selection for genetic testing" says Niloy Jewel (Jewel) J. Samadder, M.D., Gastroenterology and Hepatology at Mayo Clinic in Phoenix/Scottsdale, Arizona. "Identification of a germline predisposition can have important implications for treatment decisions, risk-reducing interventions, cancer screening and germline testing for affected patients and their close relatives."
INTERCEPT assessed germline genetic alterations among patients with solid tumor cancer who received care at Mayo Clinic Cancer Center and a Mayo Clinic Health System community practice between April 1, 2018, and March 31, 2020. During the study period, English-speaking adult patients with a new or active cancer diagnosis, confirmed pathologic diagnosis of carcinoma, and treatment in medical oncology, radiation oncology, dermatology or surgical oncology clinics were enrolled.
Patients were not selected based on cancer type, disease stage, family history of cancer, ethnicity or age. "Selection for genetic testing has traditionally been based on pathologic features of the cancer, age at diagnosis, family history of cancer and other factors stipulated in clinical practice guidelines. Few studies have compared the prevalence of germline findings in patients with cancer unselected by practice guidelines," says Dr. Samadder.
All patients viewed a standardized pretest education video and were offered additional pretest genetic counseling if desired. Germline sequencing using a next-generation sequencing panel of 83 genes (84 genes as of July 2019) on the Invitae Multi-Cancer Panel was offered at no cost. All test results were reviewed by a certified genetic counselor and disclosed to the patient, and those with PGVs were invited for genetic counseling.
The final cohort
A total of 3,095 patients were enrolled in the study:
- The average age of the final 2,984-patient cohort was 61.4 years, and 1,582 (53%) of the patients were male.
- A total of 535 patients (18.6%) had stage 0 or I disease, 477 (16.7%) had stage II disease, 593 (20.7%) had stage III disease, and 1,257 (43.9%) had stage IV disease at the time of genomic analysis.
- Race or ethnicity distribution included 159 Hispanic or Latino (5.3%), 110 African American (3.7%), and 53 Asian American (1.8%) patients.
- A family history of cancer in a first-degree relative was reported in 1,019 participants (34.1%).
Among the final cohort, 397 (13.3%) harbored 426 PGVs. The six most common PGVs were found in BRCA1 (OMIM 113705) and BRCA2 (OMIM 600185), monoallelic MUTYH (OMIM 604933), CHEK2 (OMIM 604373), Lynch mismatch repair genes, and ATM (OMIM 607585).
The incidence of germline PGVs in various tumors ranged from 7.3% for melanoma to more than 13% for several cancer types (ovarian, 20.6%; pancreas, 15.9%; colorectal, 15.3%; prostate, 13.7%; lung, 14.7%; cholangiocarcinoma, 14.5%; endometrial, 13.3%; and bladder, 14.2%).
PGVs were classified as high (relative risk greater than 4), intermediate (relative risk, 2 to 4), or low (relative risk less than 2) penetrance or recessive medically actionable variants. The variant rate by stage of cancer was similar: 13.7% in stage 0 to II disease and 13.1% in stage III to IV disease. Patients with a family history of cancer in the same organ system had a high rate of PGVs (21.8%).
In the 13.8% of the recruited population with non-white ancestry, the prevalence of PGVs was 10.9% and variants of uncertain significance was 62.2% (vs. 53.4% for patients of white ancestry).
结果:八分之一的患者具有癌症遗传易感性。

结果:八分之一的患者具有癌症遗传易感性。
普遍的种系基因检测发现 13.3%(八分之一)的患者具有致病性种系变异体。
致病性突变及其临床意义
致病性突变及其临床意义
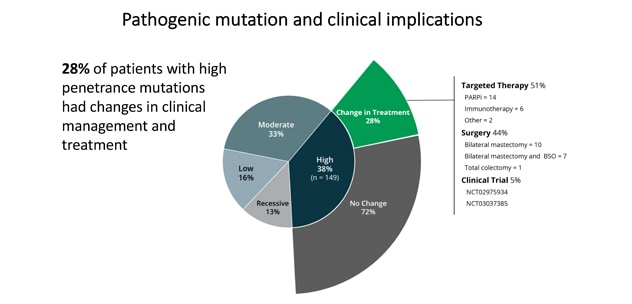
28% 的高渗透性突变患者的临床管理和治疗发生了变化。
PGVs were found in 397 patients (13.3%), including 282 moderate- and high-penetrance cancer susceptibility genes. Variants of uncertain significance were found in 1,415 patients (47.4%). A total of 192 patients (6.4%) had incremental clinically actionable findings that would not have been detected by phenotype or family history-based testing criteria. Of those with a high-penetrance PGV, 42 patients (28.2%) had modifications in their treatment based on the finding. Only younger age of diagnosis was associated with presence of PGV.
Family variant testing underused
Cascade family variant testing was offered at no cost to all blood relatives of affected participants with PGVs within a 90-day window of the patient's finalized test result report. "Surprisingly, family variant testing was pursued in less than 20% of families of probands with a PGV, even though cost was not a barrier," says Dr. Samadder, who also notes that other barriers that may have led to low uptake included limited comprehension by at-risk relatives after notification, the technical nature of genetic test reports, concerns regarding genetic discrimination and insurance coverage, and emotional burden of a cancer diagnosis.
"This study offers significant insight into the performance of multigene panel testing and has broad implications for its wide clinical implementation and acceptance in oncology practice," says Dr. Samadder. "At Mayo Clinic, steps are being taken to ensure all patients are offered genomic sequencing. The testing will help us to better understand the genes that led to the development of our patients' cancers, and how to precisely target treatment and improve survival."
Dr. Samadder presented the INTERCEPT study at the American Society for Human Genetics Virtual Meeting and it was published in JAMA Oncology in 2020.
For more information
Murphy S. Mayo Clinic study finds 1 in 8 patients with cancer harbor inherited genetic mutations. Mayo Clinic.
1427 – Universal genetic testing identifies more hereditary cancer syndrome patients than guideline directed targeted testing: A multi-center prospective study. Presentation at: American Society of Human Genetics 2020 Virtual Meeting; 2020.
Samadder NJ, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncology. In press.