Jan. 08, 2019
For patients with severe eosinophilic asthma — practically defined as patients with the need for frequent or continuous oral corticosteroid therapy or patients with inadequate asthma control despite maximal inhaler therapy — there is a new treatment option in the form of anti-interleukin-5 (IL-5) therapy.
FDA-approved anti-IL-5 therapy agents on the market
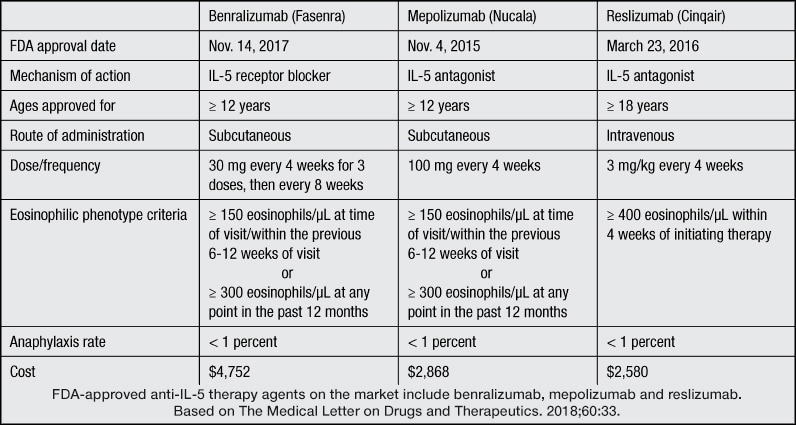
FDA-approved anti-IL-5 therapy agents on the market
There are currently three FDA-approved anti-IL-5 therapy agents on the market: benralizumab, mepolizumab and reslizumab. Results of clinical trials for these agents were published in The Medical Letter on Drugs and Therapeutics and New England Journal of Medicine between 2014 and 2018.
Both benralizumab and mepolizumab are approved for patients age 12 years and older, whereas reslizumab is approved for patients age 18 years and older. These agents appear to have excellent safety and efficacy profiles with a major beneficial impact on reduction of asthma exacerbations and associated health care utilization.
Selection of patients for anti-IL-5 therapy
It is essential that patients be carefully chosen for this step-up therapy. Anti-IL-5 therapy is approved for patients with refractory asthma with an eosinophilic phenotype. The eosinophilic phenotype is defined slightly differently for each agent.
For the purposes of mepolizumab drug approval, eosinophilia is defined by the presence of 150 or more eosinophils per microliter in the peripheral blood at the time of the clinic visit or 300 or more cells per microliter at any time during the previous year.
Patients who benefit most from anti-IL-5 therapy are those who continue to have inadequate asthma control or exacerbations despite high-dose inhaled corticosteroids, frequent oral corticosteroid use, or both.
Practice points when considering anti-IL-5 therapy
The new anti-IL-5 therapy agents have now helped to improve asthma care for many patients with severe eosinophilic asthma. The 2018 Global Initiative for Asthma (GINA) guideline recommends these therapies for severe asthma despite optimal inhaler therapy. It is important to remember that these agents are best initiated after a thorough evaluation by physicians and centers comfortable with managing patients with severe asthma.
Very rapid taper of oral corticosteroids soon after initiating anti-IL-5 therapy is not advised due to risk of an asthma flare. Patients should be advised regarding the absolute need to continue existing controller therapies for asthma, including inhaled corticosteroids, long-acting beta agonists, long-acting muscarinic antagonists and leukotriene modifiers. Compliance should be reinforced and inhaler technique verified in all patients. The small risk of anaphylaxis and need for administration in a health care facility should be discussed. In general, the anaphylaxis risk with these agents does not appear to be very different from that of omalizumab.
Proper patient selection for this new targeted therapy is important for treatment success. Mayo Clinic actively participated in several studies of anti-IL-5 therapy agents. The severe asthma care team at Mayo Clinic has developed expertise in identifying appropriate patients for this new therapy. Further research is underway to identify other potential targets for asthma monoclonal antibody therapy.
For more information
Benralizumab (Fasenra) for severe eosinophilic asthma. The Medical Letter on Drugs and Therapeutics. 2018;60:33.
Ortega HG, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. New England Journal of Medicine. 2014;371:1198.
Mepolizumab (Nucala) for severe eosinophilic asthma. The Medical Letter on Drugs and Therapeutics. 2016;58:11.
Reslizumab (Cinqair) for severe eosinophilic asthma. The Medical Letter on Drugs and Therapeutics. 2016;58:81.