Oct. 29, 2022
Patients with persistent or recurrent Cushing disease (CD) after transsphenoidal surgery (TSS) pose a difficult management dilemma. They also require additional tumor-directed therapy to reduce the metabolic consequences and physical debility related to persistent hypercortisolism. Repeat TSS offers the benefit of rapid correction of hypercortisolemia, but the rate of biochemical remission is much lower than primary operations, especially for patients with tumors extending into the cavernous sinus.
Radiation-based treatments, including external beam radiation therapy, stereotactic radiosurgery (SRS) and proton beam therapy, are frequently used as second line treatments for patients with persistent or recurrent CD. However, biochemical remission only occurs after a latency interval, and many patients develop hypopituitarism after irradiation. Pharmacotherapy for patients with adrenocorticotropic hormone (ACTH)-secreting pituitary adenomas has been used, but the relative efficacy and durability of these approaches is not optimal.
Although bilateral adrenalectomy (ADX) is safe and provides excellent control of hypercortisolemia, it is generally considered the treatment of last resort related to the risk of adrenal crisis and Nelson-Salassa syndrome, according to studies published in Annals of Surgical Oncology and the Journal of Neurosurgery.
A retrospective review of 38 patients without prior radiation treatment having SRS for ACTH-secreting pituitary adenomas from 1990 to 2015 was published in Neurosurgery. One of the study's lead authors, Bruce E. Pollock, M.D., a neurological surgeon at Mayo Clinic's campus in Rochester, Minnesota, says, "A favorable outcome was defined as biochemical remission and tumor growth control. Patients were evaluated separately if they underwent bilateral ADX. In every case, single-fraction SRS was performed.
Axial and coronal MRI
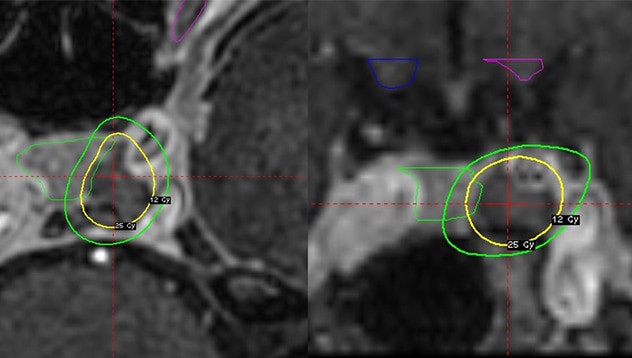
Axial and coronal MRI
Axial (left panel) and coronal (right panel) MRI showing the SRS dose plan for a 33-year-old woman with an enlarging left-sided tumor following transsphenoidal surgery and bilateral adrenalectomy. The tumor received a minimum dose of 25 Gy; the maximum dose to the left optic nerve was 8.1 Gy.
"The goal of dose planning was to provide complete tumor coverage while excluding the pituitary gland. Whenever possible a margin dose of 25 Gy or more was prescribed while limiting the maximum radiation dose to the optic nerves and chiasm to below 12 Gy," Dr. Pollock adds.
William Young, Jr., M.D., an endocrinologist at Mayo Clinic in Rochester, Minnesota, states, "Pituitary hormone assessment was performed before SRS, at six and 12 months after SRS, then yearly thereafter. The standard hormonal evaluation included measurement of serum electrolytes, ACTH, morning cortisol, free thyroxine, thyroid-stimulating hormone, insulin-like growth factor-1, testosterone levels in men, and estrogen levels, when indicated, in women."
Biochemical remission for patients having SRS alone was defined as a normal 24-hour urinary free cortisol excretion. Hypopituitarism was defined as new anterior pituitary deficits detected after SRS. Follow-up MRI was performed at six, 12 and 24 months after SRS, but the imaging frequency then decreased to every 2 to 3 years. Tumors greater than 2 mm smaller or larger in diameter were defined as decreased or increased in size, respectively.
Visual field testing was performed at six, 12 and 24 months after SRS then discontinued unless the patient had specific visual complaints. The median follow-up after SRS was 76 months (IQR, 40 to 149). Twenty patients (53%) were treated with ADX and SRS (median margin dose, 25 Gy) and 18 patients (47%) received SRS alone (median margin dose, 22.5 Gy).
Results
Coronal MRI
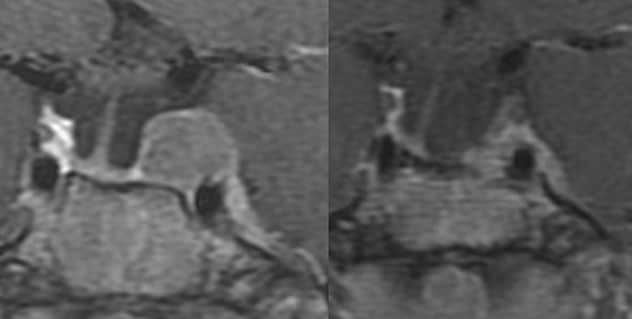
Coronal MRI
Coronal MRI of a 19-year-old woman with severe Cushing disease following 2 transsphenoidal surgeries. The patient underwent concurrent bilateral adrenalectomy and SRS. Left panel: MRI prior to SRS (margin dose, 25 Gy). Right panel: MRI four years after bilateral adrenalectomy and SRS showing a significant reduction in the tumor size.
- Eighteen of 20 patients (90%) having ADX had a favorable outcome.
- Two patients (10%) had tumor growth requiring additional treatment.
- Thirteen of 18 patients (72%) having SRS alone at a median of 14 months (IQR, 8 to 23) reached a favorable outcome.
- Five patients (28%) required additional treatment due to persistent ACTH overproduction (n = 4) or persistent ACTH production and tumor growth (n = 1).
- Eight of 34 patients (24%) with normal pituitary function or partial hypopituitarism before SRS developed new anterior pituitary deficits at a median of 24 months (IQR, 15-40) after SRS.
- The rate of new pituitary deficits was 15% at two years and 22% at five years. The risk of new deficit by hormonal axis based on the number of patients at risk was hypoadrenalism, 11% (2/18); hypogonadism, 10% (3/31); hypothyroidism, 20% (6/30); and GH deficiency, 12% (4/34).
- Two patients (6%) developed complete anterior pituitary failure.
- Four of 18 patients (22%) having SRS alone developed later deficits. One patient (5%) had two episodes of adrenal crisis in the first year after ADX requiring hospitalization secondary to dehydration and hyponatremia, but no more episodes in three additional years of follow-up.
- No other patient had an adrenal crisis in 154 years of follow-up. No patient developed visual loss, diplopia, radiation necrosis or carotid stenosis after SRS.
Dr. Pollock concludes: "SRS is an effective treatment for patients with persistent or recurrent CD. Patients with mild to moderate CD can be safely managed with SRS alone, whereas patients with severe CD should be considered for bilateral ADX with either concurrent SRS or SRS performed if tumor growth is detected."
For more information
Szabo Yamashita T, et al. Bilateral adrenalectomy: Differences between Cushing disease and ectopic ACTH-producing tumors. Annals of Surgical Oncology. 2020;27:3851.
Graffeo CS, et al. Characterizing and predicting the Nelson-Salassa syndrome. Journal of Neurosurgery. 2017;127:1277.
Hughes JD, et al. Radiosurgical management of patients with persistent or recurrent Cushing disease after prior transsphenoidal surgery: A management algorithm based on a 25-year experience. Neurosurgery. 2020;86:557.
Refer a patient to Mayo Clinic.