Dec. 28, 2021
Mayo Clinic performs MRI on individuals with a range of implanted medical devices. A multidisciplinary team that includes MRI safety officers and physicists, as well as specialized clinicians, has extensive experience with altering the MRI parameters to obtain imaging while ensuring patient safety.
"We try to be intelligently aggressive about scanning patients with devices because we know Mayo Clinic is often a last stop for many people seeking a diagnosis and treatment," says Robert E. Watson, Jr., M.D., Ph.D., a neuroradiologist at Mayo Clinic in Rochester, Minnesota, and the MRI medical director for MR safety. "We have the capability to use MR-dependent, cutting-edge technology — including MR-guided laser ablation and focused ultrasound — to treat patients who have neurological conditions such as epilepsy and tremor. We believe that we should do our best to offer these procedures to patients who have implanted devices, too."
Dr. Watson, who is chair of the American Board of Magnetic Resonance Safety and of the American College of Radiology's MRI Safety Committee, notes that Mayo Clinic's intelligently aggressive approach applies to all types of implanted devices.
"Increasingly, patients come to us with more than one device — for example, a spinal cord stimulator as well as a pacemaker," he says. "That adds complexity, but we can generally do the scan and get the answers we need for that patient."
Another challenge is knowing exactly what device a patient has. There are three designations:
- MR safe: The device contains no metal.
- MR conditional: The device contains metal and a possible energy source such as a battery. MRI can be safely performed with strict attention to parameters, such as limiting the amount of radiofrequency deposition and the magnet's field strength.
- MR unsafe: No adaptations to the scanning parameters can make MRI safe.
Scanning a patient incorrectly can lead to serious injury or even death. Great attention to detail in correctly identifying implanted devices is essential, as model numbers for MR conditional and MR unsafe devices can differ by only two digits.
While some patients are knowledgeable about their implanted devices, others are less clear. Mayo Clinic asks referring physicians to do all in their power to help identify the exact make and model.
"It's absolutely essential that we know precisely what's implanted in our patients. It's as important as knowing their medications and allergies," Dr. Watson says. "If we know what's in the patient, we can do our best to tailor the MRI to scan that patient safely."
An experienced, dedicated team
Although manufacturers are making more MR conditional devices, some patients have older implanted devices that are nonconditional and are technically MR unsafe. Mayo Clinic's multidisciplinary MRI team routinely works with referring clinicians to design protocols that allow safe scans for patients so that referring clinicians and radiologists can reach the correct diagnosis.
For patients with pacemakers considered MR unsafe, Mayo Clinic's scanning team includes cardiologists and cardiology pacing nurses. The pacemakers are programmed appropriately before and after the scans, and patients are closely monitored throughout. Pulse sequences are chosen by a radiologist and tailored by a physicist. The scans are performed by a technologist with special training in MRI for patients with implanted devices.
"We do on average four MRIs a day for individuals with pacemakers. It's become a well-oiled machine," Dr. Watson says. "Demand for this procedure at Mayo Clinic is exceptionally high."
For patients with challenging neurological conditions, Mayo Clinic's scanning expertise facilitates treatment using state-of-the-art interventional MRI. For example, laser interstitial thermal therapy (LITT) — which is increasingly used to treat some patients with mesial temporal lobe epilepsy — requires MRI to pinpoint placement of the laser probe and identify the tissue being ablated.
In a technical case report published in the November 2018 issue of Operative Neurosurgery, Mayo Clinic neuroradiologists and neurosurgeons described a protocol used to safely perform LITT on an 83-year-old patient with a nonconditional pacemaker and a history of focal dyscognitive seizures. Five months after the procedure, the patient was seizure-free and had experienced no complications.
More recently, Mayo Clinic performed MR-guided focused ultrasound for ablation of a painful lumbar facet joint in a patient with a pacemaker and an abandoned pacemaker lead. "An abandoned lead is a significant safety risk," Dr. Watson says. "However, we were able to perform the procedure without complications with the assistance of our team of radiology, neurosurgery and cardiology colleagues."
Mayo Clinic also routinely performs MRI on individuals with various types of neurostimulators and with cochlear implants. Individuals with cochlear implants might have neurofibromatosis 2, which requires periodic MRI to assess tumor progression. Magnets in some older cochlear implants can flip when subjected to the magnetic fields in MRI, causing pain.
"This is clearly an informed consent situation, balancing risk versus benefit," Dr. Watson says. "Proceeding with the cochlear implant magnet in place involves a firm, thorough wrapping of the head with gauze to lessen the possibility of a magnet flip that can cause pain. The advantage of proceeding in this way is that it avoids the need for surgical removal of the implanted magnet before MRI and replacement afterward — which risks infection as well as breakdown of the Silastic pocket in which the implanted magnet is located."
Newer cochlear implants are MR conditional, with magnets that freely rotate to align with the scanner magnet to prevent magnet flips and pain. "The science is catching up with us, thankfully," Dr. Watson says.
Mayo Clinic is at the forefront of research on MRI safety. In a review published in the November 2020 issue of Magnetic Resonance Imaging Clinics of North America, Mayo Clinic radiologists outlined safety considerations for the use of MRI in patients with implanted active devices.
Possible future use of 7-tesla MRI
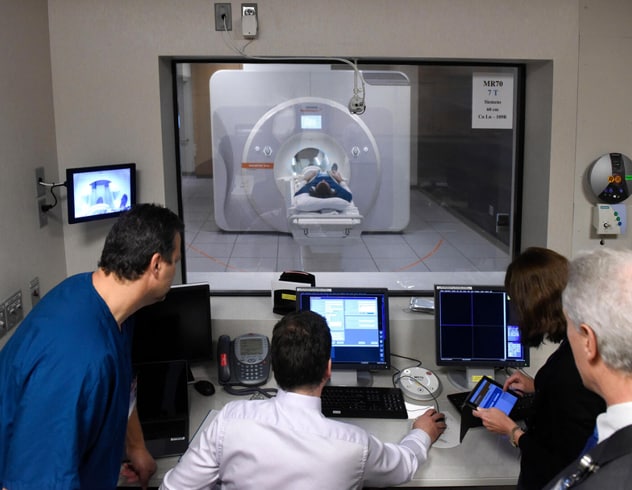
Possible future use of 7-tesla MRI
Photograph shows a 7-tesla MRI performed at Mayo Clinic. Mayo Clinic's multidisciplinary MRI team is exploring how 7-tesla MRI might be used in the future to safely scan patients with implanted medical devices.
"While totally aware of the challenges, we are delving into how to scan patients who have implanted devices on our 7-tesla magnet," Dr. Watson says. Mayo Clinic had the first clinical 7-tesla MRI scanner in North America.
The use of implanted medical devices is increasing. Mayo Clinic remains committed to safely scanning individuals with those devices whenever possible. "Patients who have implanted devices are, by definition, already sick. These are people who require MRI," Dr. Watson says. "While being smart, we try to really push the limits of what we can safely scan."
For more information
Grewal SS, et al. Safety of laser interstitial thermal therapy in patients with pacemakers. Operative Neurosurgery. 2018;15:E69.
Watson RE, et al. MR safety: Active implanted electronic devices. Magnetic Resonance Imaging Clinics of North America. 2020;28:549.