May 25, 2018
Significant advancements in medicine have prolonged the human life span in developed countries, leading to a dramatic increase in the older adult population. James L. Kirkland, M.D., Ph.D., with General Internal Medicine and Endocrinology, Diabetes, Metabolism, & Nutrition at Mayo Clinic's campus in Rochester, Minnesota, says: "This increased longevity, however, has contributed to the rise in incidence of age-associated diseases. Therefore, aging itself has been receiving more attention as the single greatest risk factor for the world's most prevalent chronic diseases.
"To combat this challenge, the geroscience research community has focused on extending health span — without necessarily altering life span — by identifying interventions that promote healthy aging with the long-term goal of postponing the onset of chronic diseases, thereby reducing economic burden and improving quality of life."
Joshua N. Farr, Ph.D., with Endocrinology, Diabetes, Metabolism, & Nutrition at Mayo Clinic's campus in Minnesota, explains: "Because the onset of several chronic diseases and comorbidities, such as cardiovascular disease, diabetes, sarcopenia and osteoporosis, tends to occur concurrently in older adults, it has been hypothesized that aging is largely controlled by genetic pathways and fundamental mechanisms conserved in evolution. Therefore, it is crucial to define the specific biochemical and molecular processes that drive natural mammalian aging, so that these threats can be targeted to defend against age-related tissue dysfunction.
"Until recently, however, these processes have remained largely elusive, which has impeded the development of novel approaches to extend health span and delay or prevent multiple age-related diseases as a group."
Although several hallmarks that represent common denominators of aging have been identified, cellular senescence, a cell fate that involves an essentially irreversible state of replicative arrest induced by various types of stress, has emerged as a promising fundamental aging mechanism that could be targeted to treat multiple diseases of aging simultaneously.
Indeed, as highlighted in an article published in 2017 in EBioMedicine, mounting evidence demonstrates that senescent cells accumulate in various tissues with aging, where they commonly develop a unique secretome of chemokines, cytokines and extracellular matrix degrading proteins, termed the senescence-associated secretory phenotype (SASP), that can actively damage the tissues in which senescent cells reside, causing natural aging phenotypes.
Dr. Kirkland highlights: "In addition, senescent cells are characterized by profound phenotypic alterations, including upregulation of the cell cycle inhibitors, p16Ink4a (Cdkn2a) and p21 (Cdkn1a), increased metabolic activity, resistance to apoptosis, telomere shortening and decondensation of pericentromeric satellite DNA. There is now strong evidence in rodents that selective elimination of senescent cells or blocking the detrimental effects of the SASP improves cardiovascular function, enhances insulin sensitivity and reduces frailty."
Drs. Kirkland and Farr and collaborators reported the first senolytic drugs — agents that selectively eliminate senescent cells — in a 2015 article in Aging Cell.
Dr. Farr explains: "In articles published in the 2016 Journal of Bone and Mineral Research and in 2017 in Nature Medicine, we reported that senescent cells accumulate with aging in the bone microenvironment, where they play a causal role in age-related bone loss. Osteoporosis, which is responsible for 2 million fractures and $19 billion in related costs every year, is characterized by decreased bone formation relative to resorption, resulting in compromised bone microarchitecture and increased risk of skeletal fractures.
"In recent studies in young versus old mice, we isolated various cell populations from the bone microenvironment, such as B cells, T cells, myeloid cells, osteoblast progenitors, osteoblasts and osteocytes. We then demonstrated that a subset of cells within each of these lineages becomes senescent with aging — although senescent osteocytes and senescent myeloid cells are the predominant culprits that produce the SASP.
"In subsequent studies in old mice, two different senolytic strategies were used to selectively kill senescent cells without affecting nonsenescent cells. These strategies included both a genetic and pharmacological approach, as well as a senomorphic approach, which blocked the pro-inflammatory effects of senescent cells using a JAK1/2 inhibitor. The studies have demonstrated that targeting senescent cells with each of these interventions for two to four months improved bone mass, microarchitecture and strength.
"Importantly, none of these interventions had any effect on bone parameters in young mice, demonstrating specificity of these approaches to aging. Comprehensive in vivo bone histomorphometry analyses revealed that targeting senescent cells led to reduced bone resorption with either maintained (within the trabecular skeletal compartment) or enhanced (along the endocortical bone surface) bone formation, demonstrating an overall anabolic effect. These findings were complemented by detailed mechanistic studies demonstrating specific factors in the SASP-impaired osteoblast mineralization, while simultaneously enhancing osteoclast progenitor survival.
Senolytic therapy eliminates senescent cells
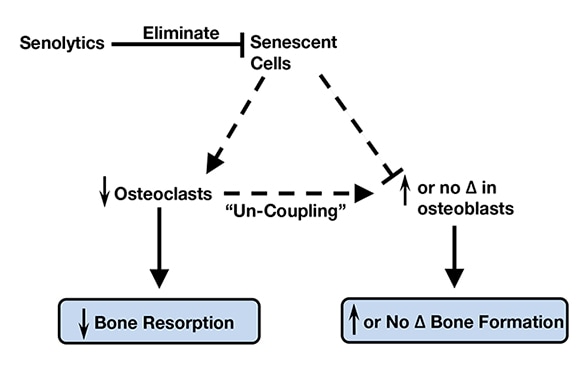
Senolytic therapy eliminates senescent cells
With aging, senescent cells accumulate in the bone microenvironment where they promote osteoclastogenesis and inhibit osteoblastogenesis. Senolytic therapy alleviates these effects by eliminating senescent cells, which in turn reduces bone resorption and either maintains (trabecular sites) or enhances (cortical sites) bone formation due to uncoupling between osteoclasts and osteoblasts. Based on Nature Medicine.
"Intriguingly, elimination of senescent cells alleviated the osteoporosis-associated accumulation of fat in bone marrow, which in combination with the observed increase in osteoblasts, suggests that senescent cell elimination causes a lineage switch whereby bone marrow mesenchymal stem cells preferentially develop into osteoblasts as opposed to adipocytes. Therefore, with aging, senescent cells accumulate in the bone microenvironment where they promote osteoclastogenesis and inhibit osteoblastogenesis. Senolytic therapy eliminates senescent cells, which subsequently reduces bone resorption and either maintains (trabecular sites) or enhances (cortical sites) bone formation due to uncoupling between osteoclasts and osteoblasts. Collectively, these data establish a causal role for senescent cells in bone loss with aging, and reveal a novel therapeutic strategy to treat osteoporosis."
Sundeep Khosla, M.D., with Endocrinology, Diabetes, Metabolism, & Nutrition at Mayo Clinic's campus in Minnesota, concludes: "Although senolytic and senomorphic therapies are now being tested in human clinical studies and although they are effective when given intermittently in rodents, there are several challenges that remain. These challenges include the theoretical concern that eliminating senescent cells could interfere with the beneficial effects of these cells. Indeed, cellular senescence is not universally detrimental, but rather can have beneficial biological functions in certain contexts.
"For example, senescent cells through their SASP can attract immune cells to sites in need of tissue regeneration and wound healing. Therefore, because targeting senescent cells is a potentially transformational strategy to extend health span, future work is needed to establish the long-term efficacy and safety of this therapeutic paradigm."
For more information
Kirkland JL, et al. Cellular senescence: A translational perspective. EBioMedicine. 2017;21:21.
Zhu Y, et al. The Achilles' heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell. 2015;14:644.
Farr JN, et al. Identification of senescent cells in the bone microenvironment. Journal of Bone and Mineral Research. 2016;31:1920.
Farr JN, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nature Medicine. 2017;23:1072.