July 16, 2021
The introduction of immune checkpoint inhibitor (ICI) drugs has revolutionized cancer therapy. The drugs target immune checkpoint molecules, such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1), that contribute to cancer pathogenesis. Immune checkpoints are closely involved in maintenance of immunological tolerance to self-antigens; ICIs have, thus, been associated with immune-related adverse events (irAEs). Endocrinopathies including hypothyroidism and hyperthyroidism, hypophysitis, primary adrenal insufficiency, and autoimmune diabetes mellitus are among the more commonly reported events. Hypoparathyroidism with ICI use is an exceedingly rare endocrine complication.
Case presentation
A 76-year-old male presented to the emergency department with one-weeklong complaints of weakness, anorexia and confusion. He started a combination immunotherapy of ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) seven months prior for widespread metastatic melanoma to the pericardium, lung, liver and lymph nodes. Two months after the initiation of this regimen, he developed irAEs of colitis and pneumonitis. His immunotherapy was put on hold. Approximately four months later, he was switched to nivolumab monotherapy. He received two infusion cycles with the last dose given 28 days prior to our evaluation.
On presentation, the patient's physical examination was remarkable for a blood pressure of 93/48 mm Hg. Chovestek sign and Trousseau sign were not present. Laboratory test results from his initial evaluation in the emergency department included:
- Total serum calcium: 5.7 mg/dL on presentation; reference range 8.8 to 10.2 mg/dL
- Serum albumin: 3.0 g/dL on presentation; reference range 3.5 to 5.0 g/dL
- Serum phosphorus: 5.1 mg/dL on presentation; reference range 2.5 to 4.5 mg/dL
- Serum magnesium: 1.7 mg/dL on presentation; reference range 1.7 to 2.3 mg/dL
- Serum ionized calcium: 3.01 mg/dL on presentation; reference range 4.57 to 5.43 mg/dL
- Serum sodium: 118 mmol/L on presentation; reference range 135 to 145 mmol/L
An electrocardiogram was remarkable for prolonged QT corrected interval of 492 milliseconds. The patient was immediately treated with a total of 3 grams intravenous calcium gluconate and normal saline infusion.
Further work-up showed an undetectable serum parathyroid hormone (PTH < 6.0 pg/mL; reference range 15 to 65 pg/mL) and a normal serum total 25-hydroxyvitamin D of 31 ng/mL.
The patient was diagnosed with primary hypoparathyroidism. Notably, he denied any history of previous neck surgery or head and neck radiation. Anti-parathyroid antibody, for which a clinically available test is performed by radiobinding assay, previously recognized in some cases of autoimmune hypoparathyroidism, was undetectable in this case.
Serum calcium, phosphate, PTH
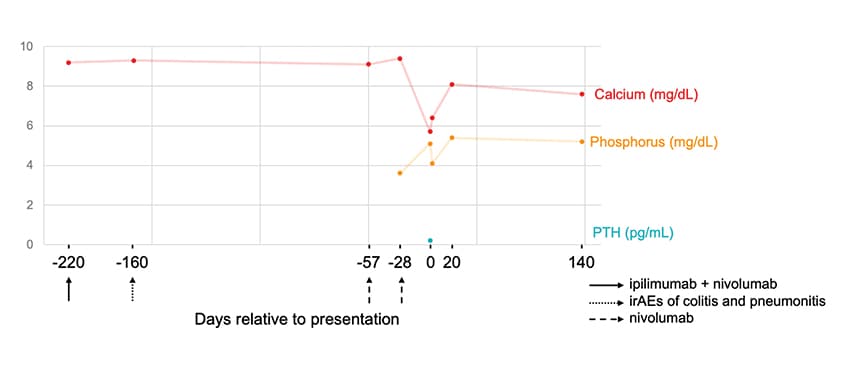
Serum calcium, phosphate, PTH
Serum calcium, phosphate and PTH levels relative to the ICI treatment regimen. PTH: parathyroid hormone; ICI: immune checkpoint inhibitor.
A review of the patient's record showed that calcium levels had been within the normal range until the index presentation, with a median total serum calcium of 9.3+/-0.23 mg/dL over a period of five years prior to any ICI therapy and median creatinine of 1.29+/-0.27 mg/dL. PTH was normal at 49 pg/mL six years prior to presentation.
Additional testing performed due to concerns for other endocrinopathies, particularly adrenal insufficiency due to noted hyponatremia, included a morning cortisol that was low at 2.0 mcg/dL (reference range, 7 to 25 mcg/dL) and a serum ACTH that was inappropriately normal at 8.3 pg/mL (reference range, 7.2 to 63 pg/mL). MRI of the brain did not reveal any pituitary or hypothalamic abnormalities. Both thyroid stimulating hormone and free T4 were normal at 2.9 mIU/L (reference range, 0.3 to 4.2 mIU/L) and 1.1 ng/dL (reference range, 0.9 to 1.7 ng/dL), respectively.
Outcome and follow-up
The patient was started on 500 mg of oral calcium carbonate (elemental calcium) three times daily and 1,000 IU of cholecalciferol daily, along with 0.25 mcg of calcitriol twice daily. His serum calcium level showed improvement over the course of three days. Hydrocortisone replacement was initiated with good clinical response in blood pressure, sodium levels and symptoms. After 22 days of starting this regimen, serum calcium was 8.1 mg/dL with serum albumin of 4.0 g/dL and a 24-hour urinary calcium of 198 mg every 24 hours.
Discussion
"Given the lack of other associated causes of hypoparathyroidism, late age of onset and absence of other autoimmune polyglandular syndrome features — combined with the temporal relationship between ICI therapy and other associated irAEs including hypophysitis with secondary adrenal insufficiency — we believe this case illustrates hypoparathyroidism as a rare complication associated with ipilimumab and nivolumab therapy," says Robert A. Wermers, M.D., chair of Endocrinology, Diabetes, Metabolism, and Nutrition at Mayo Clinic in Rochester, Minnesota.
"While this combination of drugs has been shown to improve progression-free survival in melanoma, it may be associated with an increase of irAEs compared with monotherapy. Moreover, endocrine complications present earlier, at 30 days, with combination therapy compared with 76 days for complication presentation in those treated with a single agent (ipilimumab)," Dr. Wermers notes.
The patient had also previously reported irAEs (colitis and pneumonitis), which may be of significance as a predictor of developing additional irAEs. Whether or not this patient's course of combination therapy followed by nivolumab monotherapy created a cumulative toxicity effect is difficult to ascertain based on current literature.
"The mechanism of irAE-related hypoparathyroidism remains unclear, but it is postulated that autoantibodies may play a role," says Omar M. El Kawkgi, M.B., B.Ch., B.A.O., an Endocrinology, Diabetes, Metabolism, and Nutrition fellow, at Mayo Clinic in Minnesota. "Anti-parathyroid and activating calcium sensing receptor (CaSR) autoantibodies have been implicated in autoimmune hypoparathyroidism. Indeed, activating CaSR autoantibodies were detected in a patient with primary hypoparathyroidism receiving nivolumab.
"Anti-parathyroid antibodies have been described in patients with autoimmune endocrine conditions such as Addison's disease and Hashimoto's thyroiditis, as well as cases of idiopathic hyoparathyroidism and animal models of autoimmune hypoparathyroidism. Further research is needed to elucidate the role of these various antibodies and if ICI-associated hypoparathyroidism may be due to a destructive immune process as may occur in other endocrinopathies."
Unlike other irAEs, endocrinopathies often require lifelong treatment with permanent hormone replacement. The reasons for this difference are unclear. In this case, 77 days after ICI use, PTH remains undetectable and the patient continues to require 500 mg of elemental calcium three times daily and 0.25 mcg of calcitriol twice daily to maintain his serum calcium within a satisfactory range. Persistence of hypoparathyroidism despite discontinuation of ICI drugs has been previously recognized.
Dr. Wermers concludes: "In an era of novel immuno-oncology treatments, it is important to consider ICI blockade-mediated hypoparathyroidism in patients presenting with hypocalcemia. Particular attention may be needed for patients who received combination ICI and have had previous irAEs. Future studies should focus on understanding the mechanism of ICI-associated hypoparathyroidism, predicting factors and long-term outcomes with such events."
For more information
El Kawkgi OM, et al. Hypoparathyroidism: An uncommon complication associated with immune checkpoint inhibitor therapy. Mayo Clinic Proceedings: Innovations, Quality & Outcomes. 2020;4:821.