April 16, 2014
Amyloidosis is caused by extracellular deposition of autologous protein that is laid in a conformation described as beta-pleated sheets and known as amyloid fibrils. Cardiac amyloidosis is a clinical condition in which the cardiac structures are infiltrated by beta-amyloid protein, leading to a constellation of findings on history, physical examination and cardiac imaging. The protein that is deposited has varied subtypes, with each having its own natural history.
Amyloid deposition typically occurs in a uniform pattern, but certain variants may show a more patchy involvement. These proteins are deposited in the interstitial space and on biopsy, which remains the current gold standard of diagnosis, amyloid fibrils bind Congo red stain, yielding apple-green birefringence under cross-polarized light microscopy.
Noninvasive imaging features of cardiac amyloidosis are generally sufficient to detect or at least suspect the diagnosis, and cardiac MRI has an emerging role in diagnostic evaluation. It is important, however, to note that the diagnosis is based upon the integration of several clinical features and basic testing, including laboratory testing and electrocardiogram, that lead to the clinical suspicion of the disease.
Cardiac imaging is typically performed in patients with known systemic amyloidosis presenting with cardiac symptoms or signs. Echocardiography is usually the first cardiac imaging test performed, typically because it:
- Is more widely available, portable and affordable
- Has robust and well-established techniques to measure diastolic function, which is the most important physiologic abnormality in cardiac amyloidosis
- Has developed newer applications such as strain imaging
Cardiac MRI use
Cardiac MRI is, however, emerging as a first line modality or at least a complementary imaging modality in patients with suspected cardiac amyloidosis, specifically with regard to its superior ability to characterize the myocardial tissue on post-contrast myocardial delayed enhancement sequences. A comprehensive assessment of cardiac amyloidosis using cardiac MRI requires tailored, flexible imaging protocols to complement other clinical information. Cardiac MRI has shown considerable promise in the diagnostic evaluation of cardiac amyloidosis.
Cardiac morphologic features are evaluated using steady-state free precession sequences. This particular sequence allows visualization of both cardiac structure and function. Images of the heart are taken throughout the cardiac cycle, affording the ability to determine cardiac volumes and ejection fraction. Similarly, the myocardial thickness can be assessed, including both ventricular and atrial wall and interatrial septal thickness.
These findings in the setting of low-voltage electrocardiogram should raise the suspicion of the diagnosis. The ability to image the heart in any plane is one of the major advantages over traditional echocardiographic assessment, which may be limited by the available acoustic windows.
The steady-state free precession sequence is also useful in the identification of pericardial and pleural effusions, which may be accompanying findings. Impaired diastolic function may also be apparent and, similar to the Doppler echocardiographic assessment of diastolic function, mitral inflow measurements can be obtained using cine phase-contrast pulse sequences.
One of the utilities of cardiac MRI that makes it an attractive imaging modality in patients with cardiac amyloidosis is its ability to characterize abnormalities in the tissue structure. In order to achieve this goal, though, intravenous administration of gadolinium contrast is necessary. This becomes an important consideration when selecting a patient for cardiac MRI.
The major factor precluding the use of intravenous gadolinium contrast is the presence of significant renal dysfunction; at Mayo Clinic, specialists use a cutoff for glomerular filtration rate of 30 milliliters per minute per body surface area (mL/min/BSA), and those above this threshold are considered candidates for contrast administration. This becomes a particularly important consideration in patients with amyloidosis, as these patients may commonly be afflicted by significant renal dysfunction.
After administration of contrast medium, there are often striking abnormalities of myocardial tissue characteristics that are seen on myocardial delayed enhancement pulse sequences. Patients with cardiac amyloidosis typically demonstrate diffuse and irregular hyperenhancement of the myocardium. The hyperenhancement pattern may be circumferential and subendocardial in distribution but is quite variable.
Right ventricular involvement also may be apparent on these sequences. The delayed enhancement sequence can also be useful in the identification of intracardiac thrombus, particularly intra-atrial thrombi, which may also be a feature in patients with the disease who may be presenting with stroke-like symptoms.
Myocardial nulling
One of the important distinctions that needs to be made is between cardiac amyloidosis and other nonischemic cardiomyopathies, most notably hypertrophic cardiomyopathy, that can have potentially similar imaging findings. Abnormalities in myocardial nulling are a common feature in amyloidosis and can be useful in such a distinction.
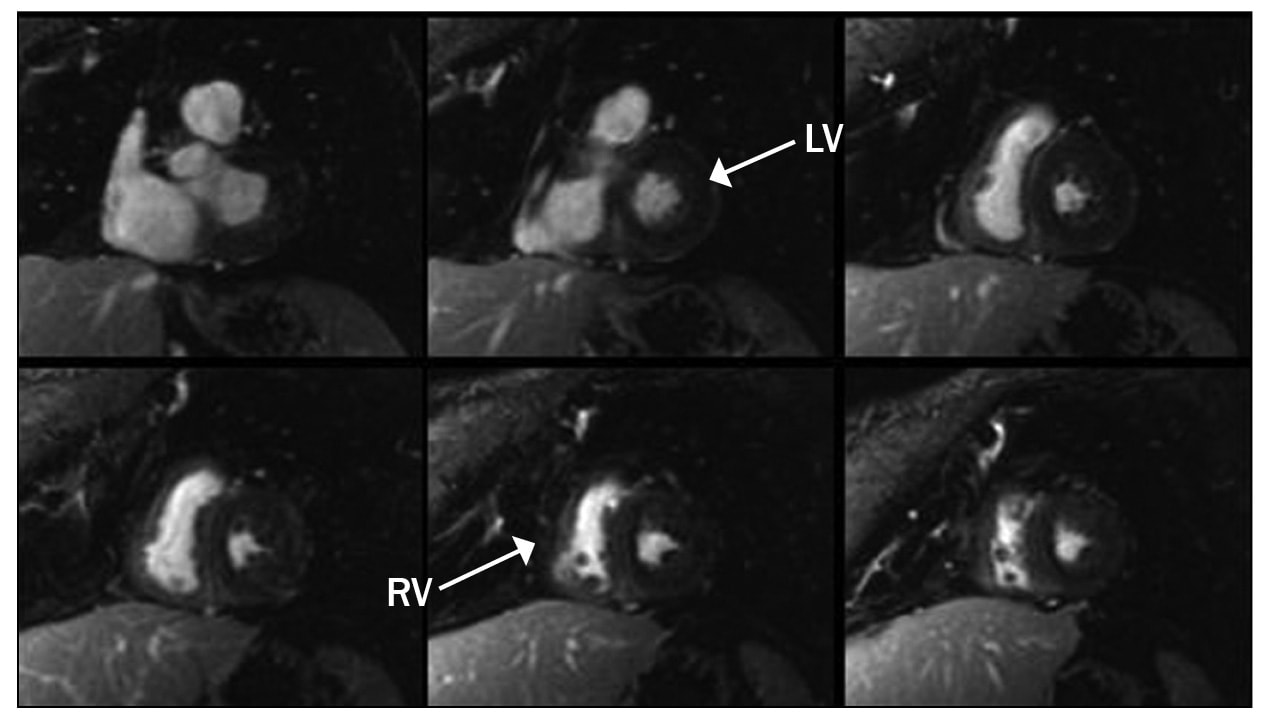
Myocardial delayed enhancement sequence in a patient with cardiac amyloidosis
Myocardial delayed enhancement sequence in a patient with cardiac amyloidosis
Images of myocardial delayed enhancement sequence in a patient with cardiac amyloidosis. Note the abnormal, diffuse enhancement; this finding is characteristic of infiltrative cardiomyopathies.
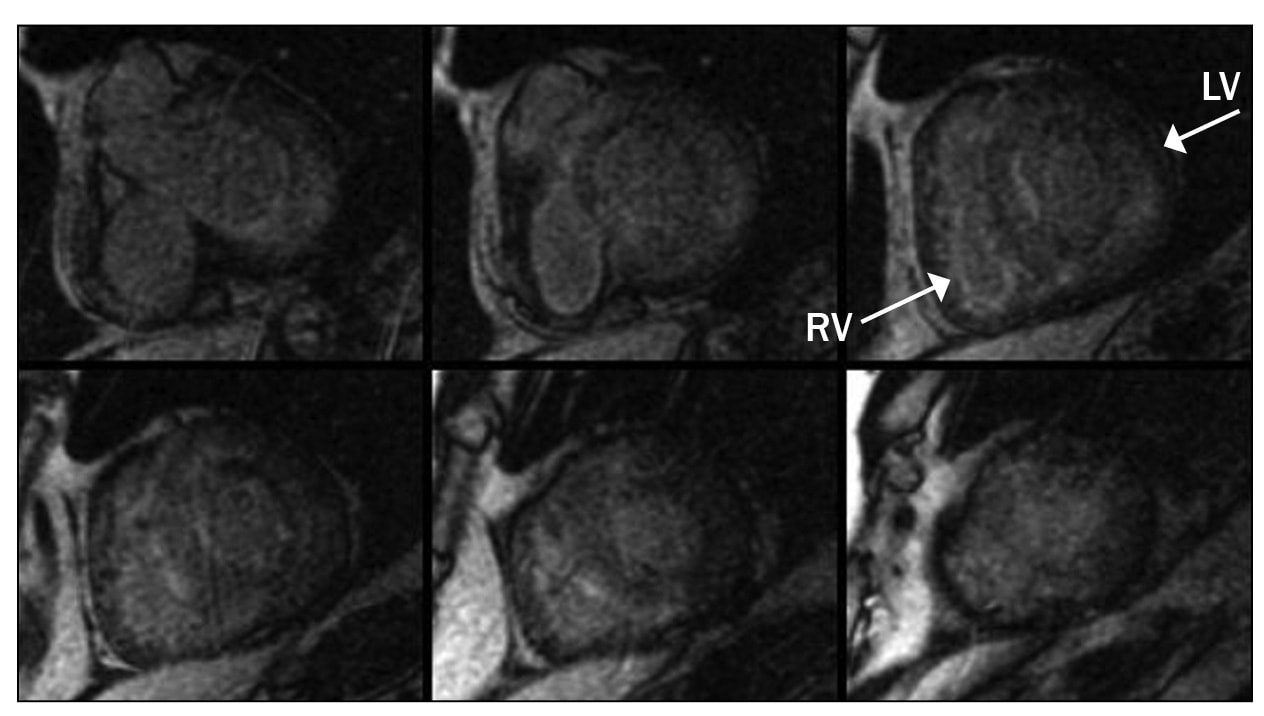
Myocardial delayed enhancement sequence
Myocardial delayed enhancement sequence
Myocardial delayed enhancement sequence. Note the nulling characteristic of the normal myocardium, which appears dark on the sequence.
The term "myocardial nulling" refers to an inversion recovery pulse sequence that is used to null the signal from a desired tissue to accentuate surrounding pathology. A common use of this technique is to null the signal from normal myocardium during delayed-enhanced imaging. The nulled normal myocardium will be dark in contrast to the enhanced abnormal myocardium. For example, in the setting of a myocardial infarction, the infarcted myocardium will appear bright next to the normally dark nulled noninfarcted myocardium.
The inversion recovery pulses have a special parameter known as the inversion time. To null normal myocardium, the MRI technician must find the appropriate inversion time at which the normal myocardium is dark. This time does vary slightly from person to person and as such is a parameter that is determined at the time of the scan.
A cine multi-inversion time inversion recovery sequence, in which each image is acquired with a slightly longer inversion time, is often used to select the optimal inversion time for the delayed enhancement acquisition. As the inversion time increases, the blood and myocardium pass through a null point at which the signal is minimized.
Generally, the blood pool contains a higher concentration of gadolinium and passes through the null point before the myocardium. In cardiac amyloidosis, however, this normal blood-pool-to-myocardium relationship is reversed and as such, the myocardium reaches the null point before the blood pool. The actual timing of the null point in the blood pool and myocardium can be graphed using on-board software contained on the imaging platform that is used in a particular institution.
The evaluation of cardiac amyloidosis is clearly multifaceted and is directed initially by patient characteristics, laboratory studies and electrocardiographic studies. Noninvasive imaging techniques, particularly cardiac MRI, are having a more central role in the evaluation of patients with cardiac amyloidosis and, when integrated with other clinical features, may lead to an efficient and cost-effective clinical evaluation.