Nov. 20, 2015
CarboMedics (29 mm) mitral valve prosthesis
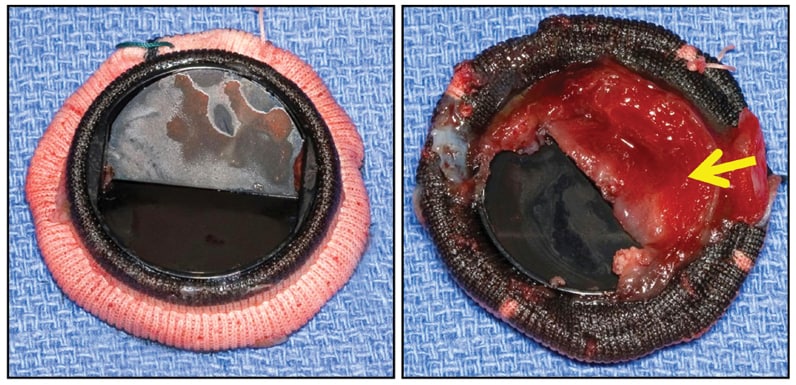
CarboMedics (29 mm) mitral valve prosthesis
CarboMedics (29 mm) mitral valve prosthesis, with large shallow thrombus (arrow) entirely covering inflow aspect of one flap, completely immobilizing it, but without obstructive fibrous ingrowth (pannus).
St. Jude porcine bioprosthesis
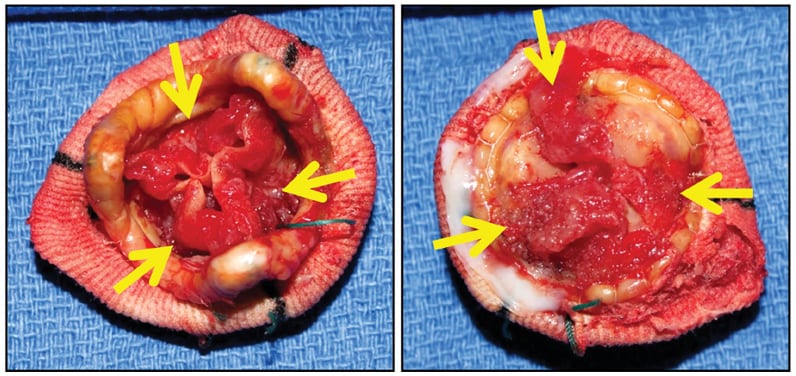
St. Jude porcine bioprosthesis
St. Jude porcine bioprosthesis, with degenerating old obstructive thrombus (arrows) along both sides of all three cusps (left, downstream side; right, upstream side). There was no acute inflammation or identifiable microorganisms.
Valve replacement with a prosthetic mechanical or tissue valve is the only treatment for many diseased cardiac valves. Thrombosis of a prosthetic valve is potentially life-threatening, resulting in hemodynamically severe stenosis or regurgitation.
"Thrombotic risk is related to the type of valve, position of the valve and adequacy of anticoagulation," according to Vuyisile T. Nkomo, M.D., M.P.H., director of the Valvular Heart Disease Clinic at Mayo Clinic in Rochester, Minnesota. "Other factors include thrombogenicity of the prosthetic material, shear stress and localized areas of abnormal flow." Therefore, individuals with mechanical valves require lifelong anticoagulation with warfarin along with aspirin, whereas those with tissue valves usually require anticoagulation for only three to six months followed by lifelong aspirin therapy.
The target international normalized ratio (INR) for individuals with bileaflet or current-generation single tilting disk mechanical aortic valves is 2.5 if they have no risk factors for thromboembolism and 3.0 if they have a ball-cage mechanical valve (because of the associated higher risk of thrombosis) or risk factors for thromboembolism such as atrial fibrillation, left ventricular systolic dysfunction, prior thromboembolism or hypercoagulable state.
The goal INR is 3.0 (range, 2.5 to 3.5) for patients with mechanical mitral valves and 3.5 to 4.0 for patients with mechanical tricuspid valves. The incidence of mechanical valve thrombosis is 0.5 to 8 percent for left-sided mechanical valves and 20 percent for right-sided valves (likely attributable to lower flow and gradient).
Although uncommon, tissue valve thrombosis can occur. Signs and symptoms of mechanical valve thrombosis may include muffled mechanical heart sounds, a new murmur, dyspnea, heart failure and cardiogenic shock. Thrombosis of right-sided valves causes right-sided heart failure, characterized by swelling of the legs, abdomen or both, without pulmonary congestion. The onset of symptoms may be progressive or acute, depending on the rate and degree of stenosis or regurgitation.
Another potential difficulty is thromboembolism resulting in transient ischemic attack, stroke or pulmonary embolism. Therefore, valve thrombosis should be suspected and immediately investigated in anyone with a mechanical valve who develops new auscultatory findings, dyspnea, heart failure, peripheral edema, thromboembolism or shock.
Thrombus, vegetation or pannus?
3-D transesophageal echocardiogram
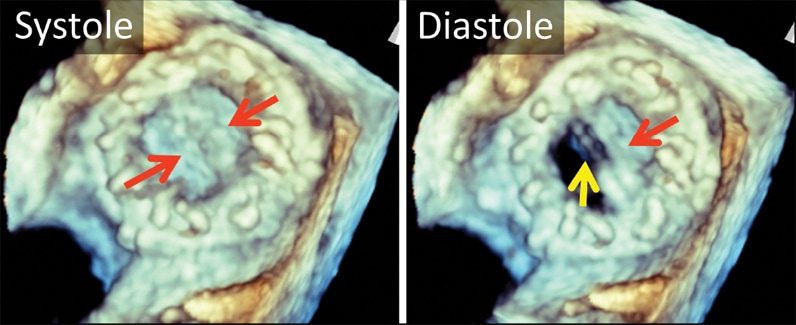
3-D transesophageal echocardiogram
3-D transesophageal echocardiogram. Left atrial view of bileaflet mechanical mitral valve prosthesis. Note both leaflets in closed position in systole (left, arrows), with opening of only one disk in diastole (right, yellow arrow). The red arrow marks the fixed disk.
2-D transesophageal echocardiogram of bileaflet mechanical mitral valve prosthesis
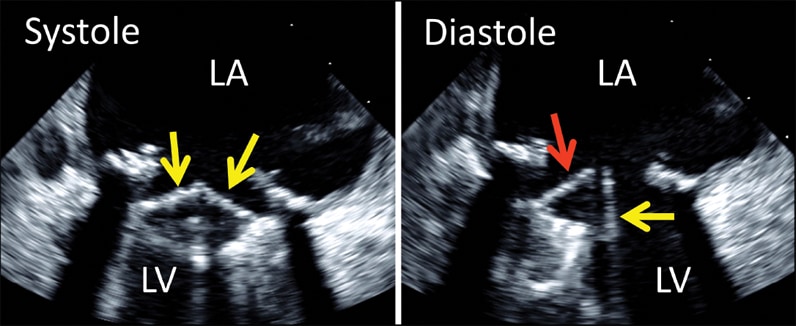
2-D transesophageal echocardiogram of bileaflet mechanical mitral valve prosthesis
2-D transesophageal echocardiogram of bileaflet mechanical mitral valve prosthesis. Note both leaflets in closed position in systole (left, arrows), with opening of only one disk in diastole (right, yellow arrow). The red arrow denotes the fixed disk. LA, left atrium; LV, left ventricle.
Both transthoracic and transesophageal echocardiography should be performed when there is suspicion of abnormal valve prosthesis function. There are some distinguishing features between thrombus and pannus in terms of when they occur after mechanical valve replacement surgery and the echocardiographic appearance. Inadequate anticoagulation with warfarin should raise suspicion of thrombus. Pannus formation usually occurs many years after mechanical valve replacement, compared with thrombus, which may occur at any time. Pannus is more commonly associated with mechanical valves in the aortic position but can occur in association with any mechanical valves. Obstruction due to pannus tends to develop slowly over time, and symptom onset is usually more gradual. Obstruction related to thrombus is typically more acute.
Transthoracic echocardiography is helpful in detecting the presence and degree of obstruction and regurgitation but is inadequate in distinguishing pannus from thrombus. This distinction can be more accurately assessed by transesophageal echocardiography.
Thickened valve leaflets and an increased gradient are suspicious for thrombosis. Visually, thrombus tends to be larger and more mobile than pannus, but the best distinguishing feature between the two is lower video intensity of the thrombus compared with pannus, which can be discerned by an experienced echocardiologist. Endocarditis should be considered when a mobile echo is seen in association with a prosthetic valve or the patient is febrile with abnormal prosthesis function.
Acoustic shadowing can impair both transthoracic and transesophageal image quality. Imaging in the cardiac catheterization laboratory with fluoroscopy or imaging with computerized tomography also may be helpful in the diagnosis of abnormal mechanical leaflet motion when imaging is not adequate with transthoracic and transesophageal echocardiography.
Treatment of valve obstruction
Mechanical valves
Management is dictated by the cause of obstruction (pannus versus thrombus), the size and the severity of symptoms. Severe mechanical valve obstruction caused by pannus requires repeat open-heart surgery to remove the pannus or to replace the valve. Options for treating symptomatic obstruction of mechanical valves from thrombus include intravenous heparin, thrombolytics or repeat surgery.
Left-sided mechanical valves
Valve obstruction from a small thrombus (less than 1 cm in diameter or 0.8 cm2 in area), recent onset (fewer than 14 days) and associated with mild symptoms (New York Heart Association, or NYHA, class I-II) can be treated initially with a trial of 24 to 48 hours of intravenous unfractionated heparin with a goal activated partial thromboplastin time (APTT) of 1.5 to 2 times the control value.
The patient should be monitored clinically, transthoracic echocardiography should be performed every two to four hours, and transesophageal echocardiography should be performed daily to assess the valve status. Improvement in valve function and gradient should be followed by warfarin and continued intravenous heparin until the INR is in the range of 3 to 4 for aortic prostheses and 3.5 to 4.5 for mitral prostheses.
If there is no improvement in valve function and gradient after 24 hours, thrombolytic therapy with recombinant tissue plasminogen activator (TPA) (10-mg intravenous bolus followed by 90-mg intravenous infusion over two hours or, alternatively, 20-mg intravenous bolus followed by 30-mg infusion over three hours) should be considered.
Successful thrombolytic therapy should be followed by warfarin and intravenous unfractionated heparin until the INR is 3 to 4 for aortic valve prostheses and 3.5 to 4.5 for mitral prostheses. Patients in whom thrombolytic therapy is partially successful (the valve gradient is improved but not normalized) present a challenge. Options include both subcutaneous unfractionated heparin twice daily to achieve an APTT of 1.5 to 2 times control and warfarin therapy with a target INR of 2.5 to 3.5 for three months.
Emergency surgery is recommended for patients with severe symptoms (NYHA class III-IV) and left-sided valve thrombosis, regardless of thrombus size. Emergency surgery is recommended if the thrombus size is large (more than 1 cm in diameter or greater than 0.8 cm2 in area), regardless of symptoms. The rationale for avoiding thrombolytic therapy with large thrombus burden is the marked increased risk of thromboembolism. Thrombolytic therapy can be considered if the risk precludes surgical intervention.
The overall risk of bleeding and thromboembolism with fibrinolytic therapy of left-sided valves is 17.8 percent and is directly related to thrombus size. Patients with small thrombi are at lower risk of complications. Contraindications to thrombolytic therapy include:
- A history of intracranial bleeding
- Recent cranial trauma
- Gastrointestinal or genitourinary bleeding within 21 days
- Hemorrhagic retinopathy;
- The presence of large, mobile thrombi
- Severe hypertension
- Hypotension or cardiogenic shock
- Major surgery within the previous two weeks
Right-sided mechanical valves
A trial of intravenous heparin followed by thrombolytic therapy (in the absence of contraindications) is the mainstay of treatment of right-sided mechanical valve thrombosis. Successful thrombolytic therapy should be followed by warfarin and intravenous unfractionated heparin until INR is in the range of 3.5 to 4.5. Partially successful thrombolytic therapy may be followed by subcutaneous unfractionated heparin twice daily to achieve an APTT of 1.5 to 2 times control or 55 to 80 seconds. Pulmonary embolus is a potential complication, although usually asymptomatic. Surgery should be considered if heparin and thrombolytic therapy fail.
Tissue valves are at much lower risk of thrombosis compared with mechanical valves. The typical clinical scenario is an increasing pressure gradient across the prosthesis, typically within the first five years after surgery, although late cases have been described. Thrombus is usually located in the cusp of the tissue prosthesis leaflets on the downstream side, and it is difficult to visualize, particularly when involving the aortic valve tissue prosthesis.
"The literature suggests that porcine tissue valves tend to thrombose at higher rates than pericardial tissue valves, but this may be because there have been so many more porcine valves implanted," according to Sorin V. Pislaru, M.D., Ph.D., a cardiologist at Mayo Clinic in Rochester, Minnesota. Transesophageal echocardiography should be considered in all patients with increased gradients in whom the transthoracic echocardiographic study is suspicious for tissue valve obstruction or degeneration.
Treatment of tissue valve thrombosis
Anticoagulant therapy with warfarin with a goal INR of 2 to 3 is effective in treating tissue valve thrombosis in the majority of patients. Heparin (unfractionated or low molecular weight) can be considered when rapid anticoagulation is desired (such as in patients with large thrombus burden, mobile thrombus, or uncertain hemodynamic status). However, patients who exhibit hemodynamic instability should be considered for emergent surgery or thrombolytic therapy.
In a special category are patients who are already on anticoagulant therapy; Mayo specialists recommend a trial of warfarin anticoagulation at a higher INR target in the range of 2.5 to 3.5. Novel oral anticoagulants have not been used in patients with bioprosthetic valves; their role in bioprosthetic thrombosis is entirely unknown, and they cannot be recommended at this time.
Summary
Cardiac valve replacement is increasingly common as a result of valvular disease in aging populations. Prompt identification of valve dysfunction and timely treatment mitigate the risk of catastrophic outcomes. Mayo Clinic cardiologists and cardiac surgeons are always available to consult by phone, review images and evaluate these patients on an urgent basis.