March 24, 2023
Mayo Clinic's Pediatric Brain Tumor Clinic is committed to finding new treatment options and ways to improve patients' quality of life. Clinician-researchers are developing targeted therapies and investigating methods of delivering treatments that boost efficacy and mitigate side effects.
"It's critical that we develop novel therapeutics and treatment methods because right now the options are limited," says Jonathan D. Schwartz, D.O., M.P.H., a pediatric neuro-oncologist at the Mayo Clinic Children's Center in Rochester, Minnesota.
The work is especially challenging due to the rarity of pediatric brain tumors and their differing biology compared with adult brain tumors. "Also, because children are a vulnerable group, we must define safety as a top priority before we study the efficacy of new pediatric treatments," Dr. Schwartz says.
Specific projects include:
- A nationwide investigation aimed at reducing cognitive impairment in children who have radiation therapy.
- An assessment of Mayo Clinic's new approach to radiation therapy for diffuse midline glioma.
- Laboratory studies of potential new therapies and drug-delivery methods.
- CAR-NK and oncolytic virus therapies.
- Artificial intelligence (AI) for more-targeted treatment.
Reducing radiation therapy's cognitive effects
Studies have shown that radiation therapy can cause steady declines in cognitive function in children who undergo that treatment for brain tumors. Mayo Clinic has led efforts to determine if memantine can mitigate those toxic effects.
"Memantine might block receptors in the brain that are known to contribute to declines in attention, memory or thought processes," says Nadia N. Laack, M.D., chair of Radiation Oncology in the Mayo Clinic Children's Center at Mayo's campus in Minnesota.
Dr. Laack is leading a pilot study that involves administering memantine to children before they start radiation therapy. That pilot study has spawned a similar 38-site national investigation.
"We are working toward a future where each individual child has fewer side effects and the best chance of cure."
Split-course radiation for diffuse midline glioma
Axial T2-weighted MRI scan with contrast
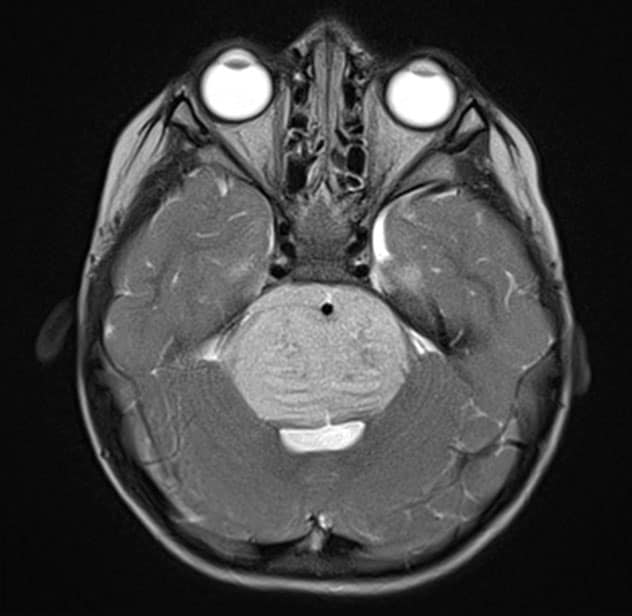
Axial T2-weighted MRI scan with contrast
Axial T2-weighted MRI scan with contrast demonstrates the typical appearance of a newly diagnosed diffuse midline glioma. The patient presented with new-onset ataxia and underwent a biopsy, which confirmed the presence of an H3KM-mutated glioma. The patient received fractionated radiation therapy.
Sagittal T1-weighted MRI scan with contrast
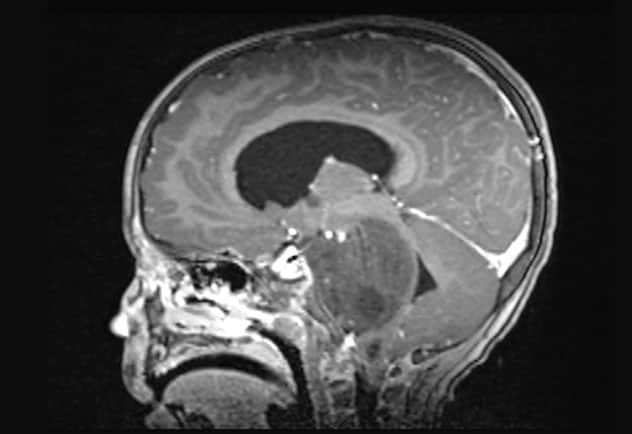
Sagittal T1-weighted MRI scan with contrast
Sagittal T1-weighted MRI scan shows the newly diagnosed diffuse midline glioma.
Reducing the toxic effects of radiation therapy is also the goal of a new Mayo Clinic protocol for children with diffuse midline glioma. That rare cancer typically occurs in young children and is fatal within a year.
Standard-of-care treatment is six weeks of radiation therapy. A second course is considered when symptoms arise or the tumor grows and other options are not available.
"But treating the same area twice with high doses of radiation comes at a cost," says Anita Mahajan, M.D., a radiation oncologist in the Pediatric Brain Tumor Clinic.
Under the new protocol, children have an initial two weeks of radiotherapy followed by close monitoring. "We delay the second two-week course until there is some evidence that the tumor is acting up. If need be, we give a third two-week round later," Dr. Mahajan says. "We still monitor and treat the tumor. But this is an opportunity to use radiation more strategically."
Mayo Clinic researchers are assessing the time intervals between patients' first and second radiation courses, as well as any effects on treatment and survival from splitting the radiation course.
Improving quality of life is the new protocol's primary goal. "Spending time at home participating in normal daily activities, instead of days in the hospital, is what makes a child's and family's quality of life better," Dr. Mahajan says. "We're going to be with our patients through anything that presents. We want to have a positive impact on their lives."
Developing and delivering drugs
Other research seeks to develop medications to treat diffuse midline glioma. One approach targets the STAT3 signaling pathway. As described in a study published in the October 2022 issue of Neuro-Oncology, researchers in the Mayo Clinic Experimental Drug and Therapeutics for Pediatric Brain Tumor Laboratory demonstrated that a small molecule inhibitor of STAT3 halted tumor growth and prolonged survival in orthotopic xenograft models.
"The STAT3 pathway is definitely important, and it appears to be druggable," says David J. Daniels, M.D., Ph.D., a pediatric neurosurgeon at Mayo Clinic Children's Center and the laboratory's director. Several STAT3 inhibitors have been developed in the laboratory for potential clinical testing.
In addition, Mayo Clinic has a National Institutes of Health grant to study the targeted drug conjugate known as GB-13. In a study published in the April 24, 2022, edition of Pharmaceutics, Mayo Clinic researchers found that GB-13 administration demonstrated a promising response in high-grade glioma models.
Devising ways to better deliver existing therapies to brain tumors is another key focus. "We have found that many drugs clear the brain too quickly to have the appropriate therapeutic effect," Dr. Daniels says.
The laboratory has developed a method of convection-enhanced delivery — which bypasses the blood-brain barrier to transport medication directly to a tumor — over an extended period of 5 to 7 days. The system has been tested in laboratory specimens. Planning is underway for a phase 1 clinical trial.
CAR-NK cells, oncolytic therapies and AI
Mayo Clinic researchers are designing chimeric antigen receptor (CAR)-engineered NK cells, which hold promise as targeted treatment for pediatric brain cancer tumors.
"CAR-NK cells are genetically engineered soldiers," Dr. Schwartz says. "They can potentially be placed in the brain carrying antibodies directed against patients' specific tumors."
CAR-NK immunotherapy avoids the release of toxic cytokines into the patient's blood. "The incidence of graft-versus-host disease is much lower compared with CAR-T cell therapy," Dr. Schwartz says.
Mayo Clinic also is investigating improvements for oncolytic virus therapies. "We are genetically modifying viruses to target cancer cells and spare healthy cells. The goal is to lessen toxicity and also attract immune cells to improve efficacy," Dr. Schwartz says.
AI projects involve the use of radiomics and radiogenomics. Extracting large quantities of data from medical imaging has potential for predicting treatment response and molecular and pathological subtypes, as well as distinguishing tumor progression from pseudoprogression.
"These are truly unmet needs in pediatric neuro-oncology," Dr. Schwartz says.
Mayo Clinic's wide-ranging research efforts bring together a multidisciplinary team of neuroradiologists, neurosurgeons, oncologists and pathologists. "We are all on board with advancing the science," Dr. Mahajan says. "We are learning so much and hope to continue to improve the way we approach pediatric brain tumors."
For more information
Pediatric Brain Tumor Clinic. Mayo Clinic.
Zhang L, et al. STAT3 is a biologically relevant therapeutic target in H3K27M-mutant diffuse midline glioma. Neuro-Oncology. 2022;24:1700.
Experimental Drug and Therapeutics for Pediatric Brain Tumor Laboratory: David J. Daniels. Mayo Clinic.
Rechberger JS, et al. IL-13R⍺2 status predicts GB-13 (IL13.E13K-PE4E) efficacy in high-grade glioma. Pharmaceutics. 2022;14:922.
Refer a patient to Mayo Clinic.