March 27, 2020
Regenerative medicine is the process of creating living, functional tissues to repair or replace tissue or organ function that is lost due to age, disease, damage or congenital defects. Mayo Clinic is careful to give patients a realistic picture of what they can expect from regenerative medicine treatments for orthopedic conditions.
Before patients receive orthobiologics at Mayo Clinic in Jacksonville, Florida, they visit the Regenerative Medicine Consult Service, part of the Mayo Clinic Center for Regenerative Biotherapeutics. There they meet with a clinical consultant who has expertise in regenerative medicine and the patients' specific conditions.
"We explain which orthobiologics we use, what the science shows about them and what outcomes we are seeing. We tell patients to please understand that regenerative medicine treatments aren't necessarily cures or substitutes for surgery," says Shane A. Shapiro, M.D., a medical orthopedist and medical director of the Regenerative Medicine Therapeutics Program at Mayo Clinic's campus in Florida. "When we educate patients, they are better able to make decisions that are right for them and their orthopedic conditions."
This effort exemplifies Mayo's commitment to evidence-based care at a time when orthobiologics are aggressively marketed to consumers and health care providers.
"Unfortunately, the field has proliferated in a way that is not always backed up by science," Dr. Shapiro says. "The excitement around regenerative medicine and stem cells has consumed both the public and even care providers who don't have the background in molecular or stem cell biology to understand what is real and what is entirely false or misleading about these therapies."
Patients' visits to the Regenerative Medicine Consult Service include medical evaluations to determine whether orthobiologics are appropriate. Misinformation about the unapproved stem cell therapy marketplace also can be corrected.
"For some patients this is an eye-opening experience, as they have received inaccurate information from websites and other sources," Dr. Shapiro says. "Infomercials disguised as educational seminars for the public are widespread. In our meetings with patients, we take care not to overemphasize the medical benefits and also to acknowledge any risks of orthobiologics."
Orthobiologic therapy at Mayo Clinic
Patients who qualify and opt for orthobiologics are treated in the Regenerative Medicine Suites, a novel, multidisciplinary surgical hybrid procedure facility for cell therapy applications. The suites are equipped for regulatory-compliant cell harvest, application, storage, receiving and quality oversight. Mayo Clinic offers procedures that involve the patient's own cells — from blood, adipose tissue or bone marrow — which are minimally processed and returned to the patient within the same surgical procedure. All clinical trials using orthobiologics are monitored by the FDA.
"Patients may experience relief of pain and improvement in function for up to a year or longer. But most procedures should not be considered a cure," Dr. Shapiro says. "All would likely need to be repeated over time — with some exceptions, such as procedures in younger patients with tendinopathies, in which cures are certainly possible." Biologic therapy is also regularly combined with best practices such as activity modification, bracing and physical therapy.
Inyección de plasma rico en plaquetas
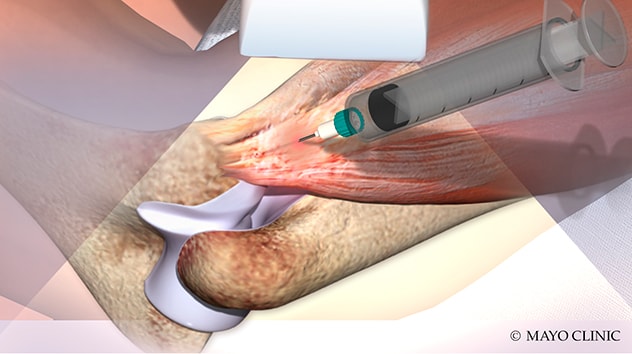
Inyección de plasma rico en plaquetas
La ilustración muestra la inyección de plasma rico en plaquetas, una de las terapias biológicas ofrecidas Mayo Clinic.
Among the orthobiologics offered at Mayo Clinic is platelet-rich plasma, which uses the patient's own platelets and growth factors to promote musculoskeletal healing. In recent years the protocols for this therapy have been refined and standardized, leading to more consistently positive results.
"We are seeing a significant clinically beneficial effect to using platelet-rich plasma in tendinopathies, as a surgical adjunct to rotator cuff repair and as pain relief for knee arthritis," Dr. Shapiro says.
Mayo Clinic also offers newer treatments using cells derived from patients' adipose tissue or bone marrow. Notably, bone marrow aspirate concentrate therapy achieved positive results in a Mayo Clinic study of patients with osteonecrosis of the femoral head. As described in the February 2018 issue of Clinical Orthopaedics and Related Research, patients with corticosteroid-induced osteonecrosis had bone marrow-derived stem cells and platelet-rich plasma injected into the femoral head after hip decompression. More than 90% of the 35 hips treated in the preliminary series avoided collapse at a minimum of two years after surgery.
"We don't necessarily view any of these first-generation orthobiologics — blood, adipose tissue or bone marrow — as definitely better than standard-of-care orthopedic surgery," Dr. Shapiro says. "For example, patients with severe knee arthritis are probably still best served with knee replacement surgery. But for patients who aren't good candidates for knee replacements, and haven't responded to physical therapy or to steroid or hyaluronic acid injections, orthobiologics can fill that treatment gap."
 Tableta de registro hace un seguimiento de los resultados autoinformados de los pacientes
Tableta de registro hace un seguimiento de los resultados autoinformados de los pacientes
La fotografía muestra la tableta de registro de resultados utilizada por los pacientes de Mayo Clinic para hacer un seguimiento de sus resultados autoinformados después de las visitas al Programa Terapéutico de Medicina Regenerativa.
After visiting the Regenerative Medicine Consult Service, about 10% of patients opt for a cell-based therapy, 25% choose platelet-rich plasma therapy, and the remaining 65% stick with standard-of-care steroid injection, physical therapy or surgery. Whichever approach patients choose, Mayo Clinic tracks treatment outcomes using validated patient-reported measures.
Additional research is underway to refine orthobiologic treatments. Dr. Shapiro is conducting a randomized clinical trial comparing therapy with adipose-derived stromal vascular fraction cells to a saline placebo for the treatment of knee arthritis. That trial builds on previously conducted randomized controlled trials, including a study published in the October 2019 issue of Cartilage. Orthopedic surgeons at Mayo Clinic in Rochester, Minnesota, have undertaken a trial of a stem cell technique, known as RECLAIM, to repair knee cartilage.
"We recognize that our first-generation orthobiologics just scratch the surface in terms of using cells to treat orthopedic disease. The future of orthopedic cell therapy is going to require much more sophisticated versions of these cell therapies," Dr. Shapiro says. "Treatment using orthobiologics that's not based in sound orthopedic science is not likely to help people. Mayo Clinic is committed to advancing the science of regenerative medicine, to harness its potential and to provide evidence-based treatments for patients."
For more information
Houdek MT, et al. Stem cells combined with platelet-rich plasma effectively treat corticosteroid-induced osteonecrosis of the hip: A prospective study. Clinical Orthopaedics and Related Research. 2018;476:388.
Shapiro SA, et al. Quantitative T2 MRI mapping and 12-month follow-up in a randomized, blinded, placebo controlled trial of bone marrow aspiration and concentration for osteoarthritis of the knees. Cartilage. 2019;10:432.