Jan. 08, 2019
Pleural diseases remain a common and challenging clinical problem. With an estimated 1.5 million new pleural effusions diagnosed annually in the United States, the incidence approaches that of diabetes (1.8 million new diagnoses annually) and eclipses that of congestive heart failure (400,000 new diagnoses annually). Spontaneous and iatrogenic pneumothoraces affect an additional 40,000.
Yet in spite of pleural diseases' high prevalence and standardized diagnostic approaches, the etiology of up to one-quarter of effusions remains unknown even after an exhaustive investigation. Almost 75 percent of effusions are ultimately due to congestive heart failure, malignancy or infection, and with an aging population, the incidence of all three continues to rise worldwide. This update addresses some of the latest understanding and approaches to management of malignant pleural disease and pleural space infection.
Malignant pleural disease
In 2010, the British Thoracic Society (BTS) published its comprehensive approach to pleural diseases. Included in these guidelines was a well-written and logical approach to the management of malignant pleural disease. Recognizing that malignant pleural effusions remain just one of a multitude of explanations for dyspnea, cough and chest pain in the setting of underlying cancer, BTS proposed in algorithmic form an approach to treating symptomatic malignant pleural effusions. Options discussed included:
- Do nothing
- Perform serial thoracentesis
- Progress on to chemical pleurodesis or tunneled pleural catheter placement
Since the overwhelming majority of malignant pleural effusions recur and require treatment within 30 days of diagnosis, BTS' recommendation for serial thoracentesis was that it be reserved primarily for patients with highly responsive malignancies or very brief life expectancy, or patients who decline other options. Life expectancy in the setting of malignant pleural effusions has been challenging to estimate, however, with performance scores the only reasonable, albeit marginally accurate, predictor.
Results of a multicenter prospective study published in Thorax in 2014 greatly advanced the ability to predict life expectancy in this group of patients. Investigators from the United Kingdom prospectively analyzed mortality in patients with malignant pleural effusions and developed and validated a scoring system conveniently named the LENT prognostic score. This four-part score assigned relative values to measurements of pleural fluid lactate dehydrogenase (L), ECOG performance score (E), serum neutrophil-to-lymphocyte ratio (N) and tumor type (T).
LENT prognostic score calculation

LENT prognostic score calculation
Pleural fluid lactate dehydrogenase (L), ECOG performance score (E), serum neutrophil-to-lymphocyte ratio (N) and tumor type (T), or LENT, prognostic score calculation. ECOG: Eastern Cooperative Oncology Group, LDH: Lactate dehydrogenase.
In the study, patients with low composite scores (0-1) were shown to have median survivals of 319 days, those with moderate scores (2-4) showed survivals of 130 days, and those with high scores (5-7) showed survivals of 44 days. For patients with high scores, more conservative management such as serial thoracentesis or tunneled pleural catheter placement may be most appropriate, whereas patients with low and moderate scores likely can be treated with any one or a combination of approaches.
For patients with low to moderate LENT prognostic scores, the 2010 BTS recommendation is to proceed to chemical pleurodesis and reserve the use of tunneled pleural catheters to those patients with unexpandable lung or failed pleurodesis.
Much has changed since the BTS guidelines were published in 2010. Of particular importance is the 2012 TIME2 trial published in JAMA. In this randomized prospective trial, patients were randomized to either undergo chemical pleurodesis with talc or placement of a tunneled pleural catheter for management of their malignant pleural effusion. The primary outcome measured was a visual analog score for dyspnea at 42 days after the intervention, which did not differ between the chemical pleurodesis and pleural catheter arms, although the dyspnea score favored the pleural catheter at six months.
Other key findings were a statistically shorter initial hospital length of stay and the need for fewer additional procedures, both favoring the pleural catheter. There were, however, more adverse events in the pleural catheter arm relative to the pleurodesis arm, although most of the adverse events were considered nonserious. This study has in many ways altered the primary approach in the management of malignant pleural effusions away from chemical pleurodesis and in favor of pleural catheters.
A subsequent retrospective review of the TIME2 data set, published in Chest in 2014, determined that up to six months there was no difference in cost between patients treated with chemical pleurodesis and those treated with tunneled pleural catheter; however, for patients with less than 14 weeks estimated survival, tunneled pleural catheters are the less costly option by a mean cost difference of about $1,700. Certainly the cost can be further reduced in those patients who achieve a spontaneous pleurodesis from the catheter itself. This outcome is known to occur in approximately 40 percent within 50 days of catheter placement and is facilitated by daily drainage.
A randomized controlled study performed in the United Kingdom and published in The American Journal of Respiratory and Critical Care Medicine in 2017 compared patients who received talc administration via the pleural catheter with those receiving placebo. The study was performed to investigate the possible role of talc pleurodesis via the tunneled catheter administered in the outpatient setting.
Although the incidence of pleurodesis in the talc-plus-pleural catheter arm (43 percent) was similar to that reported in prior studies that did not employ talc along with a pleural catheter, it significantly exceeded that in the placebo-plus-pleural catheter arm (23 percent). Participants who received talc also reported better quality of life and symptoms scores than those receiving placebo.
Pleural space infection
Pleural space infections, whether parapneumonic effusion or frank empyema, are the most common cause of exudative pleural effusions worldwide. The incidence of and mortality from pneumonia and parapneumonic effusions continue to rise.
The cornerstone of treating parapneumonic effusions and empyema remains appropriate antibiotic selection based on local microbiology and resistance patterns and establishing early drainage. Delayed drainage often results in fibrinous septation of the pleural space, which makes subsequent drainage more difficult or even impossible.
Traditionally, surgery has been needed to address undrained pockets of infected pleural fluid. However, more recently intrapleural thrombolysis has been employed with good results. The MIST2 trial results, published in the New England Journal of Medicine in 2011, demonstrated a 77 percent reduction in surgery for those patients receiving three days of twice daily intrapleural tissue plasminogen activator (TPA) and DNase. Those patients receiving this treatment also had a shorter hospital stay. However, no mortality benefit could be demonstrated based on the study design.
The study treatment included 10 mg of TPA administered into the pleural space and then allowed to dwell for one hour before drainage and subsequent administration of 5 mg of DNase. This treatment was administered twice daily for three days. Although the two medicines were administered separately for the purposes of the MIST2 trial, subsequent smaller reports published in Journal of Bronchology & Interventional Pulmonology in 2018 indicate that co-administration is likely as effective and certainly easier. This therapy is expensive (approximately $7,000 for the complete three-day treatment), and questions remain about which patients are most likely to benefit from it relative to a surgical intervention.
RAPID risk categories
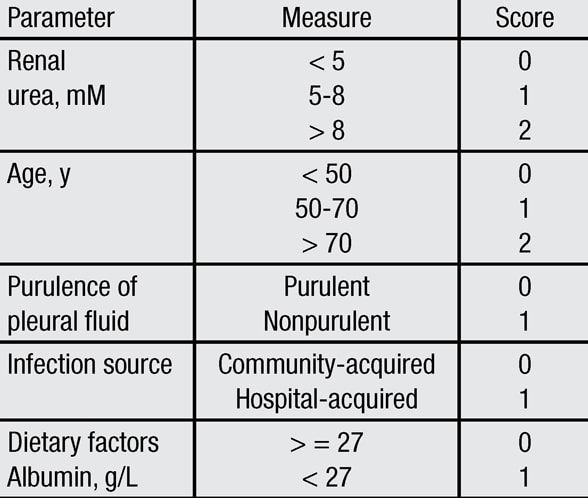
RAPID risk categories
Renal function (R), age (A), pleural fluid purulence (P), infectious source (I) and dietary factors as reflected in serum albumin (D), or RAPID, risk categories. Score 0-2: Low risk. Score 3-4: Moderate risk. Score 5-7: High risk. IQR: Interquartile range.
To help clarify this issue, the MIST2 investigators performed a retrospective review of the MIST1 and MIST2 databases and developed a multistep scoring system to better predict prognosis in those patients who present with pleural space infections. The RAPID score assigns points to various clinical measures such as renal function (R), age (A), pleural fluid purulence (P), infectious source (I) and dietary factors as reflected in serum albumin (D). By calculating this score, patients can be stratified into those at low risk (score 0-2), moderate risk (score 3-4), and high risk (score 5-7) for mortality within the subsequent three months. The study was published in Chest in 2014.
On the basis of the calculated score, those in the low-risk category were shown to have a 1 to 3 percent chance of dying within three months, whereas those in the moderate-risk group were shown to have a 9 to 12 percent chance, and those in the high-risk group, a 31 to 51 percent chance. By assessing this mortality risk, investigators believe that patients can be more appropriately stratified toward a more aggressive, early surgery arm vs. a less aggressive approach that may include intrapleural thrombolytic therapy.
For more information
British Thoracic Society Pleural Disease Guideline Group. BTS Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii1.
Clive AO, et al. Predicting survival in malignant pleural effusion: Development and validation of the LENT prognostic score. Thorax. 2014;69:1098.
Davies HE, et al. Effect of an indwelling pleural catheter vs. chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: The TIME2 randomized controlled trial. JAMA. 2012;307:2383.
Penz ED, et al. Comparing cost of indwelling pleural catheter vs. talc pleurodesis for malignant pleural effusion. Chest. 2014;146:991.
Wahidi MM, et al. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions: The ASAP trial. The American Journal of Respiratory and Critical Care Medicine. 2017;195:1050.
Rahman NM, et al. Intrapleural use of tissue plasminogen activator and DNase in pleural infection. New England Journal of Medicine. 2011;365:518.
Kheir F, et al. Concurrent versus sequential intrapleural installation of tissue plasminogen activator and deoxyribonuclease for pleural infection. Journal of Bronchology & Interventional Pulmonology. 2018;25:125.
Rahman NM, et al. A clinical score (RAPID) to identify those at risk for poor outcome at presentation in patients with pleural infection. Chest. 2014;145:848.