June 27, 2020
胶质母细胞瘤细胞外囊泡与白细胞结合
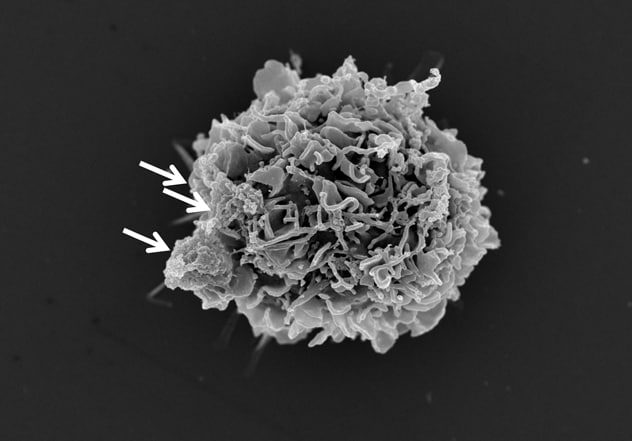
胶质母细胞瘤细胞外囊泡与白细胞结合
人胶质母细胞瘤(脑肿瘤)细胞外囊泡(白色箭头)与正常人白细胞(单核细胞)结合。这种相互作用会改变白细胞,使其抑制免疫反应。
In the fight against glioblastoma, extracellular vesicles are a doubled-edged sword. On the one hand, glioblastoma-derived extracellular vesicles appear to be important culprits in suppressing patients' immune systems. But the tumor cell-released particles might also prove useful in the diagnosis and treatment of the disease. Mayo Clinic is shedding light on both edges of the sword, paving the way to improved glioblastoma management.
"Extracellular vesicles play a role in the biology of glioblastoma tumor growth. But they also have certain factors that we can exploit to better care for patients," says Ian F. Parney, M.D., Ph.D., a neurosurgeon and director of the Neurosurgical Oncology Laboratory at Mayo Clinic in Rochester, Minnesota.
Glioblastoma is the most common and deadly brain tumor, with a median survival of just 14 months, and novel approaches are needed. Immunotherapies have had limited efficacy, as patients with glioblastoma exhibit profound immunosuppression.
"Here at Mayo, we are looking at many different therapies to stimulate the immune system to fight glioblastoma," Dr. Parney says. "Extracellular vesicle pathways that mediate immunosuppression could be targeted alongside existing immunotherapies, such as checkpoint inhibitors and vaccines, to achieve greater benefit."
A complex pathway
Recent studies have proposed that glioblastoma-derived extracellular vesicles directly inhibit patients' T lymphocytes. "Our results suggest the process is more complicated," Dr. Parney says.
In a study to be published in Neuro-Oncology, Mayo Clinic research showed that extracellular vesicles work through monocytes — converting them into immunosuppressive cells that then inhibit T lymphocytes.
"Those results fit with what we already know about the biology of glioblastoma," Dr. Parney says. "Glioblastoma tumors are heavily infiltrated by monocytes. Almost 30% of the cells in some glioblastomas aren't tumor cells but monocytes subverted by the tumor to be immunosuppressive."
In addition to subverting monocytes, extracellular vesicles appear capable of co-opting standard cancer therapies to actually increase immunosuppression. Interferon gamma is normally a marker of a positive immune response. But tests in the Neurosurgical Oncology Lab found that treating glioblastoma cells with interferon gamma increased the immunosuppressive properties of extracellular vesicles.
"Similarly, standard treatments such as radiation and chemotherapy also make extracellular vesicles more potently immunosuppressive, at least in the test tube," Dr. Parney says. "Unfortunately, even if treatment generates an immune response, the tumor can shut it down."
Mayo's increased understanding of extracellular vesicles is bringing potential therapeutic targets into focus. The Neurosurgical Oncology Lab is investigating markers of specific types of extracellular vesicles that boost immunosuppression.
"We're hot on the trail of a few markers," Dr. Parney says. "If we can block the release of those specific types of extracellular vesicles, we could also block the immunosuppressive capacity. That could be a future target of glioblastoma treatment."
Diagnostic and delivery vesicles
The advantageous aspects of extracellular vesicles start with their potential to provide a liquid biopsy for glioblastoma. In a study published in January 2020 in the Journal of Neuro-Oncology, Mayo Clinic identified a panel of microRNAs in extracellular vesicles that are dysregulated in glioblastoma and detectable in blood. MRI — the standard means of assessing tumor response — can't clearly differentiate actual tumor growth from pseudoprogression following treatment.
"A blood test that can detect glioblastoma-derived extracellular vesicles would conceivably distinguish between tumor and inflammation. That would be very helpful," Dr. Parney says.
A liquid biopsy might even prove possible for the initial diagnosis of glioblastoma. Recent work in the Neurosurgical Oncology Lab found that the microRNAs in glioblastoma-derived extracellular vesicles contain a signature. "The signature tracks back to the tumor itself. That allows us to distinguish quite well between plasma from a patient with glioblastoma and from a healthy donor," Dr. Parney says.
The researchers also found that the microRNA signature in the plasma of a patient with glioblastoma reemerges when the tumor recurs. "That information could potentially help us track glioblastoma treatment," Dr. Parney says.
He notes that extracellular vesicles might eventually serve as delivery vehicles for glioblastoma treatment. Mayo Clinic has clinical-grade glioblastoma tumor cell lines and a current good manufacturing practice facility. "It's not a big jump to say we could genetically modify those clinical-grade tumor cells so that their extracellular vesicles deliver a gene therapy," Dr. Parney says.
Boosting existing immunotherapies
Although single-agent immunotherapies haven't so far proved rewarding against glioblastoma, Mayo Clinic sees synergy from combining immunotherapy with standard treatments. Adding therapies that target extracellular vesicle pathways might further boost treatment response.
Knowledge of extracellular vesicles might also provide guidance on the optimal timing of combined treatment. "For example, if we know that the immune response is depleted right after chemotherapy, we might then try to block the immunosuppressive pathway that is mediated through extracellular vesicles," Dr. Parney says.
As immunotherapies evolve, Mayo Clinic is focused on translating laboratory research into new treatments. "Glioblastoma-mediated immunosuppression is complex, and extracellular vesicles exert multiple effects," Dr. Parney says. "But targeting these particles looks like a fruitful strategy to help prevent immunosuppression in glioblastoma."
For more information
Himes BT, et al. The role of extracellular vesicles and PD-L1 in glioblastoma-mediated immunosuppressive monocyte induction. Neuro-Oncology. In press.
De Mooij T, et al. Short non-coding RNA sequencing of glioblastoma extracellular vesicles. Journal of Neuro-Oncology. 2020;146:253.
Neurosurgical Oncology Laboratory: Ian F. Parney. Mayo Clinic.