April 11, 2017
Chemical structure for metformin
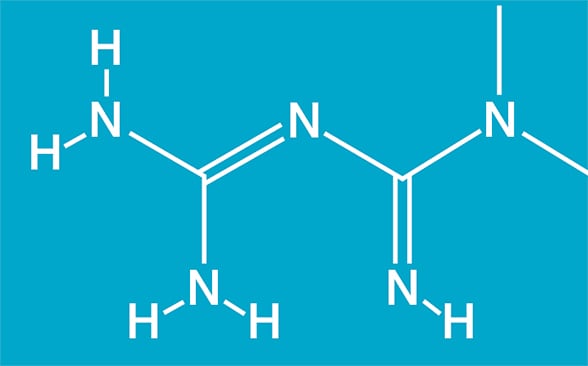
Chemical structure for metformin
Chemical structure for metformin (1,1-dimethylbiguanide; C4H11N5). Based on Cell Reports.
Metformin is the most extensively used oral therapeutic agent for type 2 diabetes mellitus (T2DM). The American Diabetes Association recommends metformin as the first line treatment for T2DM in conjunction with rigorous physical activity and dietary restriction.
K Sreekumaran Nair, M.D., Ph.D., with Endocrinology, Diabetes, Metabolism & Nutrition at Mayo Clinic's campus in Rochester, Minnesota, says: "With the rapidly expanding prevalence of T2DM, many novel oral therapeutic agents are emerging, but metformin continues to dominate, both as monotherapy as well as in combination with other medications, including insulin. Metformin can also prevent or delay the onset of T2DM in susceptible populations, such as those with prediabetes, fasting hyperglycemia or impaired glucose tolerance, and it is a safe treatment for pregnant women with gestational diabetes.
"In women with polycystic ovarian syndrome, metformin is effective not only at improving insulin sensitivity, but also in enhancing their potential for fertility. Currently, over 150 million people worldwide are using metformin."
Metformin is a highly desirable therapy for T2DM for a number of reasons. Haleigh A. James, M.D., an endocrine trainee in Endocrinology, Diabetes, Metabolism & Nutrition at Mayo Clinic's campus in Minnesota, notes: "One of its major advantages is that metformin does not cause significant hypoglycemia. Another advantage is that, unlike hypoglycemic agents such as sulfonylureas or insulin, metformin treatment is not associated with weight gain, but may cause modest weight loss.
"Some reports indicate that metformin is associated with preferential fat loss, and it may impart mild anorexic effects via its hypothalamic actions. Although there are conflicting reports, metformin may reduce the risk of cardiovascular events, especially in patients with T2DM who are overweight. This beneficial effect may be in part due to a modest effect of metformin on reducing blood pressure (unrelated to weight loss), improving lipid profiles (especially triglycerides) and endothelial function, reducing fibrinogen levels, and possibly increasing fibrinolysis."
Metformin's most common side effect is gastrointestinal distress, which includes nausea, diarrhea and upper abdominal discomfort.
Dr. Nair explains: "These symptoms are more likely to occur when patients ingest metformin on an empty stomach and may be mitigated by taking metformin in the middle of the meal or using a sustained-release formulation. The exact reasons for the gastrointestinal adverse effects are not fully understood, but there is evidence that local serotonin production may be stimulated by metformin in the gut. Slow-release metformin does not cause a rapid increase in the blood metformin levels, and a similar effect may occur when taking metformin during a meal.
"Another concern about metformin is the potential for lactic acidosis. Metformin use started in France in 1957, but it was not introduced in the United States until 1995, nearly 20 years after the biguanide phenformin was taken off the market because of its risk of lactic acidosis, which was often fatal."
Metformin has about 24 times less reported incidents of lactic acidosis compared with phenformin. Dr. James highlights: "There are many reasons why metformin causes less lactic acidosis than phenformin. It is a less powerful inhibitor of mitochondrial respiration, which is probably the main reason for its decreased risk of lactic acidosis compared with phenformin's and buformin's. Moreover, metformin increases lactate oxidation and does not increase the release of lactate from muscle, unlike phenformin.
"Metformin, however, can cause lactic acidosis in conditions where lactic acid production is high and the disposal of lactic acid is reduced. In conditions such as circulatory failure, sepsis, and anoxia or hypoxia, metformin use may result in lactic acidosis and should be avoided. It should also be avoided in patients who have renal failure with creatinine clearance below 30 mL/min/1.73 m2 or hepatic failure. Metformin interacts with some medications, including cimetidine because its metabolism is partially inhibited by metformin, thereby increasing cimetidine concentration."
Although metformin has been used for almost five decades, its mechanism of action is not fully understood. Dr. Nair highlights: "Human studies indicate the mechanistic hypoglycemic action of metformin is its inhibition of hepatic glucose production, but the underlying mechanism for this inhibition of gluconeogenesis is not fully understood. Preclinical studies in rodents demonstrated that metformin acts by inhibiting endogenous glucose production by limiting the use of glucose precursors for gluconeogenesis. Another preclinical study reported that metformin acts by inhibiting glucagon-induced hepatic glucose production. All of these studies involve rodent models with either suprapharmacological doses of metformin or other biguanides, or injected metformin directly in to peritoneum."
Results of a study performed at Mayo Clinic to determine whether these rodent experiments can be translated into humans was published in Cell Reports in 2016. Dr. Nair explains: "This study was a double-blind, placebo-controlled, randomized crossover design in patients with prediabetes to determine the effect of two weeks of metformin administration. The study confirmed that metformin increases glucose tolerance and insulin sensitivity, but it also increases plasma glucagon levels, not only in the fasted state in some study participants, but also following a meal, which seemed to prevent hypoglycemia.
"This study also indicated that metformin does not inhibit glucagon-stimulated endogenous glucose production, but in fact hyperglucagonemia may mitigate the ability of the metformin to lower endogenous glucose production, thus preventing hypoglycemia in these individuals with prediabetes. During metformin therapy, increased glucagon levels prevented a fall in endogenous glucose production, thus providing a valid explanation for why metformin administration usually is not associated with hypoglycemia. Additionally, we found that gluconeogenesis precursors were reduced by metformin as opposed to reduced utilization of glucose precursors unlike as reported in rodent models. Metformin also counteracted some of glucagon's catabolic effects, such as increased energy expenditure and protein catabolism.
Maintenance of normal blood glucose concentrations
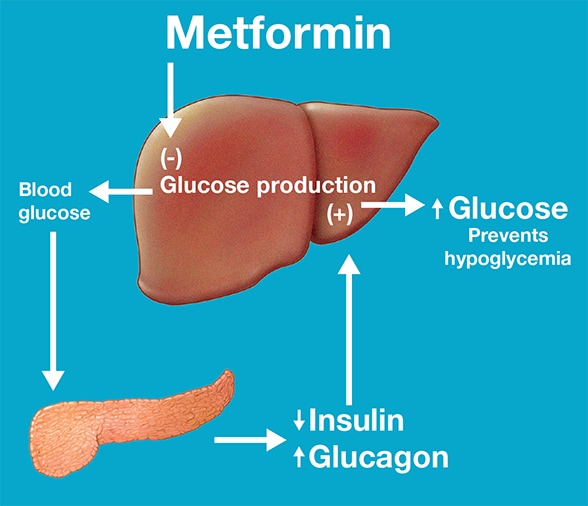
Maintenance of normal blood glucose concentrations
Maintenance of normal blood glucose concentrations in individuals with prediabetes during treatment with metformin. Based on Cell Reports.
This study thus offered insight into the effects of metformin in individuals with prediabetes. While extrapolating this information to patients with T2DM may need further clinical studies, it is likely that lack of hypoglycemia in patients with T2DM treated with metformin is explained by enhanced hepatic glucose production due to increased glucagon secretion. The study also shows that metformin reduces insulin secretion, which may reflect lesser need of insulin since insulin sensitivity is enhanced by metformin."
For more information
Konopka AR, et al. Hyperglucagonemia mitigates the effect of metformin on glucose production in prediabetes. Cell Reports. 2016;15:1394.